There are minor symptoms that technically are due







![Opioids • induce long-lasting alterations in the nervous system • [changes are] responsible for Opioids • induce long-lasting alterations in the nervous system • [changes are] responsible for](https://slidetodoc.com/presentation_image/1bc06be9e35401efc3ef0288548550aa/image-8.jpg)









































- Slides: 59



• There are minor symptoms that technically are due to cessation of the use of a drug (e. g. , the coffee drinker's early morning precoffee lethargy or minor headache). • Withdrawal is commonly, but not invariably, associated with the dependence syndrome.

• The signs and symptoms of withdrawal vary with the specific class of drug. • The severity of withdrawal is related to the amount of the substance used and the duration and patterns of use.

• Withdrawal is seen not only when the use of the substance is stopped but also when reduced use, a change in metabolism, or the administration of an antagonist results in lower levels of the drug at the relevant sites of action.

Withdrawal state in ICD-10 • There must be clear evidence of recent cessation or reduction of substance use after repeated, and usually prolonged and/or high dose, use of that substance. • Symptoms and signs are not accounted for by a medical disorder unrelated to substance use, and not better accounted for by another mental or behavioral disorder.

Opioid withdrawal was first recognized in 1700
![Opioids induce longlasting alterations in the nervous system changes are responsible for Opioids • induce long-lasting alterations in the nervous system • [changes are] responsible for](https://slidetodoc.com/presentation_image/1bc06be9e35401efc3ef0288548550aa/image-8.jpg)
Opioids • induce long-lasting alterations in the nervous system • [changes are] responsible for the physical dependence that causes an aversive withdrawal syndrome when CNS opioid levels decline

Other drug-induced changes that may persist for some time after withdrawal: • hyperresponsiveness to stress • a reduced responsivity for ordinary pleasurable events (hypophoria) • a persistent memory for the conditions under which opioids were used

• The constellation of symptoms includes multiple affective/cognitive and bodily systems reflecting the widespread neuroadaptive changes throughout the brain and body, induced by opioid administration.

Behaviors in withdrawal state • purposive behaviors: can be associated withdrawal, which are dependent on the observer and environment (e. g. , complaints, pleas, and manipulations directed at getting more drug) • nonpurposive behaviors (e. g. , piloerection and dilated pupils), which are not goal oriented

Opioid withdrawal • an opioid is no longer ingested • it is taken in smaller quantities (spontaneous withdrawal) • is acutely displaced from its receptor (precipitated withdrawal) by another drug with less activity at the opioid receptor (e. g. , an antagonist such as naloxone [Narcan]).

• All signs and symptoms of opioid withdrawal are not uniformly present across withdrawal episodes or across all individuals—there can be considerable variability between persons in the particular cluster of symptoms exhibited during opioid withdrawal.

The severity of opioid withdrawal can vary depending on: • individual differences • the pharmacologic characteristics of the opioid, such as its potency, degree of opioid agonism (e. g. , full versus partial agonist), dose, the duration of use, and the rate at which the opioid is removed from the receptors (e. g. , a slow taper versus sudden abstinence).

• The higher the potency, agonism, and dose of opioid, the longer the duration of opioid use, and the faster the removal of opioid from the receptor, the more intense the withdrawal syndrome.

Opioid withdrawal state – Dysphoric mood – craving for an opioid drug – rhinorrhea or sneezing – lacrimation – muscle aches or cramps – abdominal cramps – nausea or vomiting – diarrhea – pupillary dilatation – piloerection, recurrent chills, or sweating – tachycardia or hypertension – yawning – restless sleep – Fever – insomnia

• All signs and symptoms of opioid withdrawal are not uniformly present across withdrawal episodes or across all individuals

• The higher the potency, agonism, and dose of opioid, the longer the duration of opioid use, and the faster the removal of opioid from the receptor, the more intense the withdrawal syndrome.

In the least severe cases or early in withdrawal: • symptoms may consist only of dysphoria, irritability, restlessness, and general achiness, with few objective signs • craving, anxiety, dysphoria, yawning, perspiration, lacrimation, rhinorrhea, and restless and broken sleep

In more severe cases: • additional signs and symptoms that may be observed include increasingly dilated pupils, piloerection (waves of gooseflesh, from which comes the term cold turkey to describe withdrawal), and hot and cold flashes with visible diaphoresis

In severe syndromes: Nausea Vomiting Diarrhea weight loss fever (usually low grade) increased blood pressure, pulse, and respiratory rate • twitching of muscles and kicking movements of the lower extremities • • •

substantial subjective distress can develop before the more obvious physical signs appear.

• opioid-dependent patients in early withdrawal may exaggerate the severity of distress in the hope that it will elicit higher doses of drugs for relief.

• Although the opioid withdrawal syndrome can be exquisitely uncomfortable and distressing, it is not, in contrast to withdrawal from alcohol or sedatives, life threatening in healthy adults. However, deaths have occurred during abrupt opioid withdrawal in debilitated patients with other medical disorders.

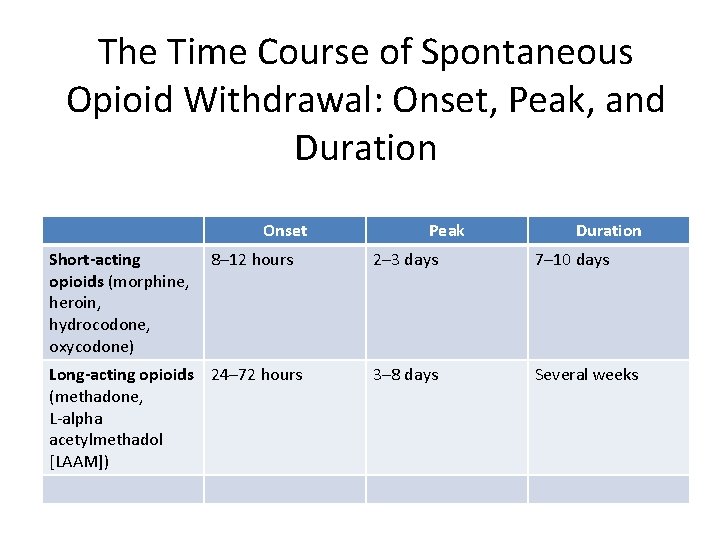
• The time course of opioid withdrawal signs and symptoms appear, peak, and dissipate depending predominantly on the half-life of the opioid.

The Time Course of Spontaneous Opioid Withdrawal: Onset, Peak, and Duration Onset Short-acting opioids (morphine, heroin, hydrocodone, oxycodone) 8– 12 hours Long-acting opioids 24– 72 hours (methadone, L-alpha acetylmethadol [LAAM]) Peak Duration 2– 3 days 7– 10 days 3– 8 days Several weeks

protracted abstinence syndrome • Following the acute opioid withdrawal syndrome • a protracted period of physiological abnormality that lasts for months • hypophoria, irritability, mood instability, decreased capacity to tolerate stress, and recurrent urges to use opioids

protracted syndrome results from • opioid-induced alterations in endogenous opioid peptide systems, opioid receptors, or other intracellular proteins that are produced in neurons exposed to opioids over long periods as well as changes in the stress response system.

• It is frequently assumed that the protracted abstinence syndrome plays a significant role in relapse to opioid use.

Treatment of Opioid Withdrawal There are now three medications approved in the United States for use in the treatment of opioid withdrawal: • Methadone • Buprenorphine • There have been some successful reports of tramadol use for opioid withdrawal.

Treatment of Opioid Withdrawal, cont. Methadone: • suppressing signs and symptoms of opioid withdrawal • a safe medication when used under clinical supervision during withdrawal • outpatient or inpatient

inpatient treatment with methadone • The primary goal of inpatient treatment is to provide doses of methadone adequate to suppress withdrawal symptoms. • the initial dosage of methadone is usually 10 to 20 mg orally. • If objective signs of withdrawal persist after the first dose of methadone, the dose can be repeated after approximately 2 hours.

inpatient treatment with methadone a general rule: • initial stabilization does not require more than 40 mg of methadone during the first 24 hours

inpatient treatment with methadone • inpatients typically stabilize on 40 to 60 mg per day or less. • After 24 to 48 hours of dose stabilization, the patient's methadone dose can be gradually reduced (for example, by reducing the dose by 10 to 20 percent per day or by 5 mg per day). • With close supervision and supportive resources, inpatient withdrawal with methadone can be accomplished in 7 to 10 days.

Outpatient treatment with methadone Goals: • alleviate withdrawal • a dose sufficient to block the effects of illicit opioids and patients' desire to use illicit opioids

Outpatient treatment with methadone • Initial dosing in the first day should begin with 20 to 30 mg and should not exceed 40 mg. • Over the first several days or even weeks of treatment, the dose may be raised (generally by no more than 10 -mg increments every 2 to 3 days) until cessation of illicit opioid use occurs.

Outpatient treatment with methadone • Once a period of stabilization has occurred— that is, no withdrawal symptoms and no illicit opioid use—then dose reductions can occur. • gradual dose reductions (e. g. , 3 percent per week) have a greater likelihood of success versus more rapid dose reductions (e. g. , 10 percent per week).

Outpatient treatment with methadone • Dose reductions are generally well tolerated, but as the dose is reduced below 20 to 30 mg per day, there is increasing withdrawal distress and increased risk of illicit opioid use. • Outpatients appear to have better outcomes when they are informed of the rate of methadone withdrawal.

Outpatient treatment with methadone Relapse is common after detoxification.

Outpatient treatment with methadone • The longer the detoxification period, the better the outcome for the detoxification episode.

Buprenorphine • sublingual tablets • two formulations: A monotherapy product (Subutex) and a combination therapy product that includes naloxone (Suboxone). • two buprenorphine dosage strengths: A small tablet (2 mg) and large tablet (8 mg)

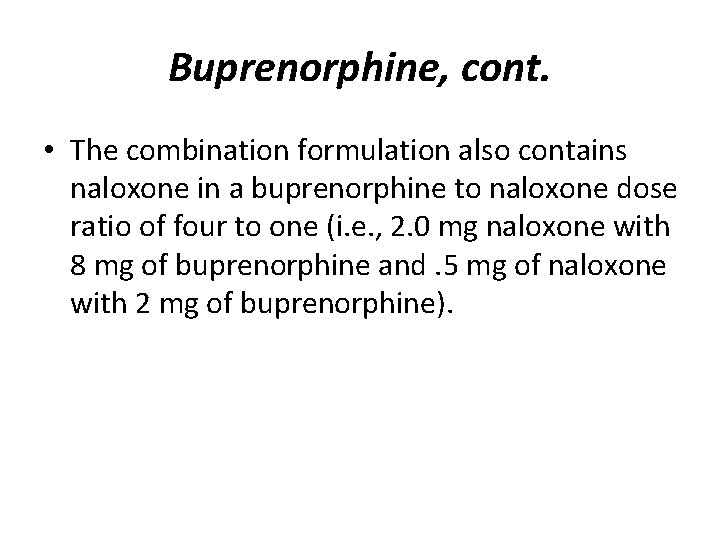
Buprenorphine, cont. • The combination formulation also contains naloxone in a buprenorphine to naloxone dose ratio of four to one (i. e. , 2. 0 mg naloxone with 8 mg of buprenorphine and. 5 mg of naloxone with 2 mg of buprenorphine).

Buprenorphine, cont. • Naloxone has little bioavailability by the sublingual route. It was added to the combination tablet to decrease the risk of diversion and misuse of the medication because if the medication is misused and injected, naloxone will be 100 percent bioavailable and may precipitate opioid withdrawal in a person who is physically dependent.

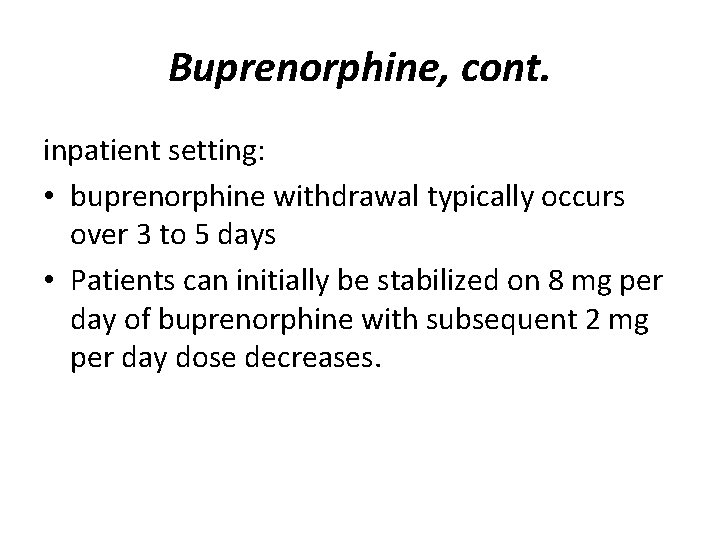
Buprenorphine, cont. inpatient setting: • buprenorphine withdrawal typically occurs over 3 to 5 days • Patients can initially be stabilized on 8 mg per day of buprenorphine with subsequent 2 mg per day dose decreases.

Buprenorphine, cont. • Buprenorphine has higher patient acceptability than clonidine and is generally well tolerated. • it is possible that because it has a relatively long duration of action, evidence of withdrawal distress from rapid inpatient detoxifications may not be evident until several days after the patient leaves the hospital.

Buprenorphine, cont. outpatient setting: • An initial stabilization dose should be achieved that is associated with objective opioid abstinence, and subsequent dose decreases should then occur gradually. • The stabilization dose will vary between patients, but will likely fall within the range of 8 to 32 mg per day.

Buprenorphine, cont. The first day's dosing should occur under medical supervision because buprenorphine is a partial opioid agonist, and the first dose can precipitate mild opioid withdrawal symptoms under certain circumstances.

Buprenorphine, cont. • To minimize the risk of buprenorphineprecipitated withdrawal, it is recommended that the first dose be given when the patient is in mild spontaneous withdrawal (12 hours after the last dose of a short-acting opioid or 24 hours after a long-acting opioid like methadone).

Buprenorphine, cont. • total dose on the first day: not greater than 8 mg • Subsequent daily dose increases of 2 to 4 mg are recommended to relieve opioid withdrawal and initiate opioid abstinence.

Buprenorphine, cont. • Subsequently, dose decreases can occur. As the smallest incremental dose of buprenorphine is 2 mg, and tablets are not scored and do not break easily, dose reductions should occur in 2 -mg increments over a period of several weeks. • Like methadone, more gradual tapers are recommended.

Buprenorphine, cont. important to note: • concurrent treatment with sedating drugs— and, most especially, benzodiazepines—is strongly discouraged in patients being treated on an outpatient basis with buprenorphine (or methadone) because of risk of significant respiratory depression if the patient takes more than what was intended or misuses these drugs by parenteral routes.

Tramadol • Doses typically totaled 600 mg on day 1 • tapered over the following 3 to 5 days • it has only been studied among inpatients in opioid withdrawal. • associated with seizures • serotonin syndrome

α 2 Agonists: Clonidine • neither are approved for this indication in the United States • low doses of opioids for example, 30 to 40 mg of oral methadone a day can abruptly discontinue opioid use

helpful in reducing • Sweating • Cramps • Nausea • Vomiting • diarrhea

• • • less helpful in alleviating muscle aches Lethargy Insomnia restlessness Craving

• starting at doses of 0. 1 to 0. 3 mg three to four times a day • In outpatient settings, a total dosage of greater than 1 mg per day is not recommended. • Hypotension • sedation

contraindicated in • acute or chronic cardiac disease (e. g. , aortic insufficiency) • renal or metabolic disorders • hypotension

• no more than 3 days of clonidine should be prescribed to an outpatient for opioid withdrawal because of the potential for overdose and death.
