THERAPEUTIC HYPOTHERMIA AND HIE Update on Diagnosis and























































- Slides: 78

THERAPEUTIC HYPOTHERMIA AND HIE Update on Diagnosis and Management 2018 Lauri Wenninger, BSN, MSN, NNP Avera Mc. Kennan Hospital

Objectives Discuss pathophysiology of HIE List eligibility and exclusion criteria related to cooling Indications and contraindications Discuss cooling during transport Safety concerns Transport protocols Become exposed to current research studies Discuss long term outcomes


Therapeutic Hypothermia Application dates back to over 5000 years Hipocrates advised snow and ice packing to reduce hemorrhage in the wounded Also used to treat tetanus Anne Greene, 1650, survived hanging in cold weather 1700 s, Dr. James Currie, Scottish physician, carried out human experiments to measure effects of cold on resp, pulse, and temp. Napoleon’s surgeon, Baron de Larrey, in 1812 packed limbs in ice prior to amputations to render procedure painless

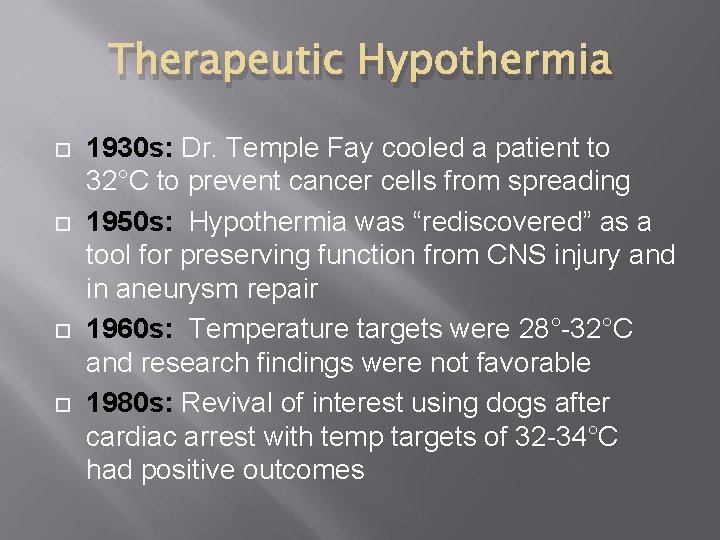
Therapeutic Hypothermia 1930 s: Dr. Temple Fay cooled a patient to 32°C to prevent cancer cells from spreading 1950 s: Hypothermia was “rediscovered” as a tool for preserving function from CNS injury and in aneurysm repair 1960 s: Temperature targets were 28°-32°C and research findings were not favorable 1980 s: Revival of interest using dogs after cardiac arrest with temp targets of 32 -34°C had positive outcomes

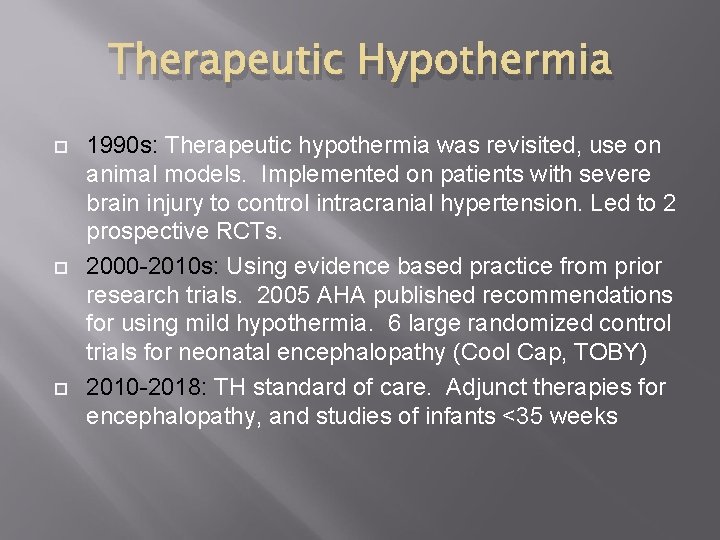
Therapeutic Hypothermia 1990 s: Therapeutic hypothermia was revisited, use on animal models. Implemented on patients with severe brain injury to control intracranial hypertension. Led to 2 prospective RCTs. 2000 -2010 s: Using evidence based practice from prior research trials. 2005 AHA published recommendations for using mild hypothermia. 6 large randomized control trials for neonatal encephalopathy (Cool Cap, TOBY) 2010 -2018: TH standard of care. Adjunct therapies for encephalopathy, and studies of infants <35 weeks

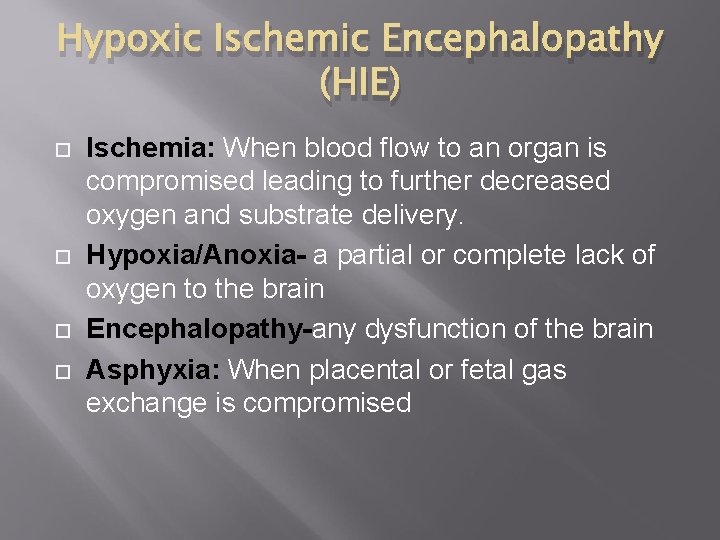
Hypoxic Ischemic Encephalopathy (HIE) Ischemia: When blood flow to an organ is compromised leading to further decreased oxygen and substrate delivery. Hypoxia/Anoxia- a partial or complete lack of oxygen to the brain Encephalopathy-any dysfunction of the brain Asphyxia: When placental or fetal gas exchange is compromised


HIE Estimated to occur in 1 -4/1000 births Neonatal HI is the most common cause of death and disability in human neonates accounting for 23 % of infant mortality worldwide and affecting 0. 7 -1. 2 million infants annually. In developed countries the incidence of HI injury has not decreased in the past two decades remaining a significant cause of fatality and disability The frequency of motor and cognitive disorders linked to perinatal and early postnatal brain injury actually increased during the 1990 s and remains stable. Among survivors, rates of disability remain high. 5 -10% motor deficits, 20 -50% sensory or cognitive abnormalities. CP one of the most costly disabilities because of its frequency (2/1000) Millar, et al 2017

Risk Factors Associated with Asphyxia Antepartum Risk Factors IUGR Severe pre-eclampsia Post dates Abnormal appearance of placenta Diabetes Intrapartum Risk Factors Maternal fever/chorioamnionitis Emergency C/S Primary hypoxic-ischemic event (abruption/uterine rupture, tight nuchal cord/compression, cord prolapse, previa, maternal hypotension) Postpartum Risk Factors Neonatal respiratory failure (severe RDS, MAS, pneumonia) PPHN A low perfusion state (septic shock) Cyanotic CHD Maternal drug/pharmacotherapy Genetic/developmental brain malformation or tumor **NOTE: potential for overlap exists

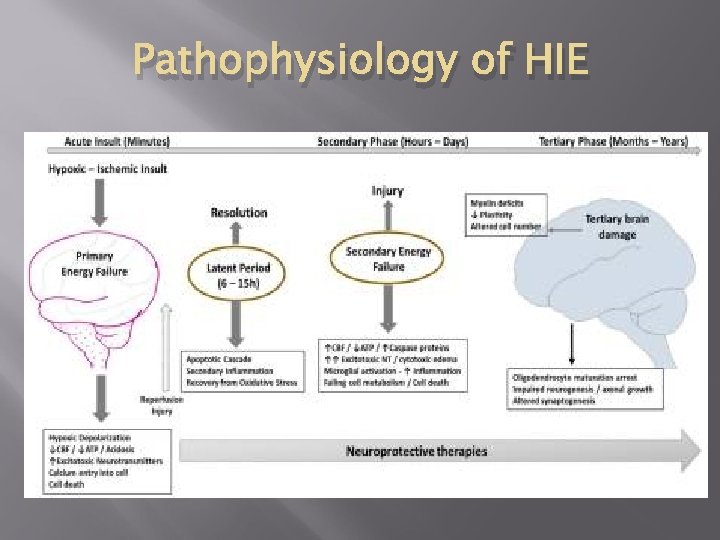
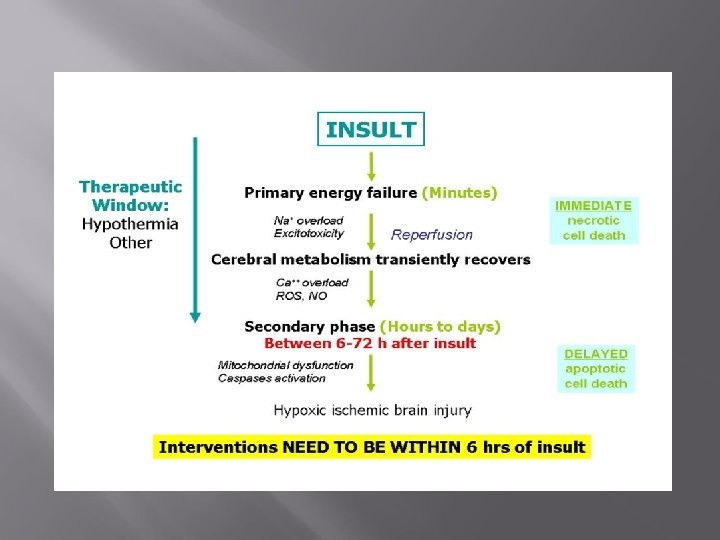
Pathophysiology of HIE Not static but evolving Initial hypoxic-ischemic insult (transient) Primary energy failure –decreased blood flow Recovery (resuscitation) Reperfusion injury Latent Period (6 -15 hours): apoptotic cascade, secondary inflammation, recovery from oxidative stress) Secondary Energy failure (6 -48 hours later) Tertiary brain damage (continues for months to years after the injury)

Pathophysiology of HIE

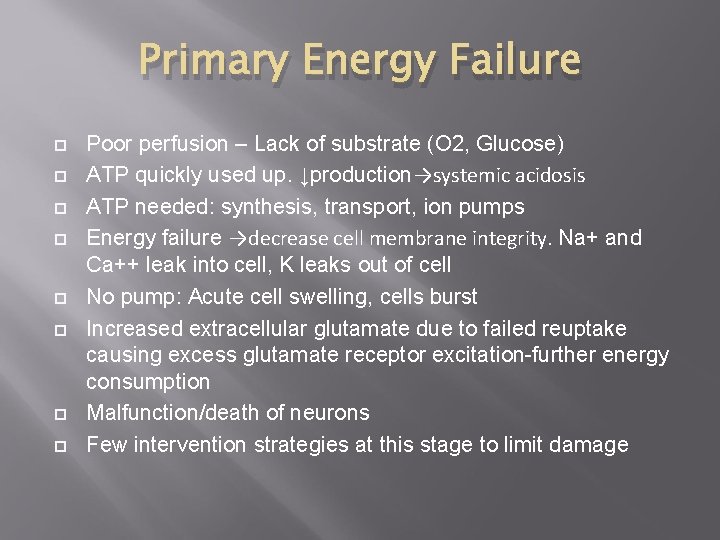
Primary Energy Failure Poor perfusion – Lack of substrate (O 2, Glucose) ATP quickly used up. ↓production→systemic acidosis ATP needed: synthesis, transport, ion pumps Energy failure →decrease cell membrane integrity. Na+ and Ca++ leak into cell, K leaks out of cell No pump: Acute cell swelling, cells burst Increased extracellular glutamate due to failed reuptake causing excess glutamate receptor excitation-further energy consumption Malfunction/death of neurons Few intervention strategies at this stage to limit damage

Resuscitation →Latent Period Restored CBF, glucose and O 2 delivery Reperfusion injury Patient encephalopathic Precedes onset of seizures in animal models Early injury cascades initiated: Inflammation Excitotoxicity Increased Ca++ O 2 radicals Apoptosis initiation: mitochondrial dysfunction

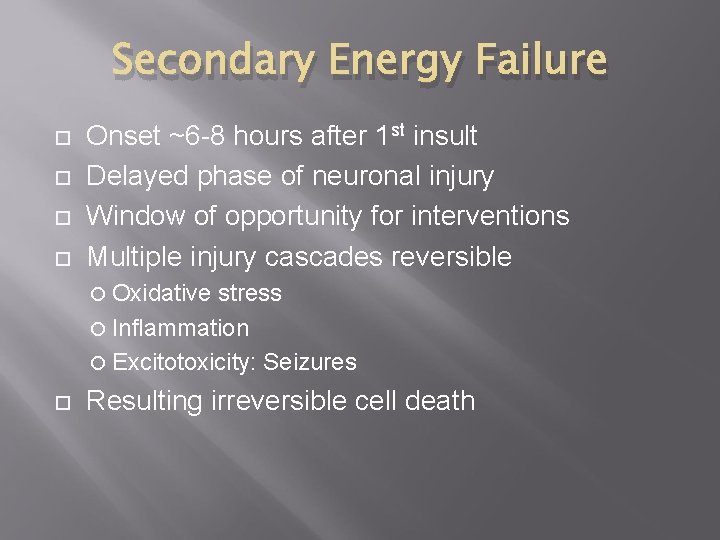
Secondary Energy Failure Onset ~6 -8 hours after 1 st insult Delayed phase of neuronal injury Window of opportunity for interventions Multiple injury cascades reversible Oxidative stress Inflammation Excitotoxicity: Seizures Resulting irreversible cell death

Tertiary Brain Damage Persist for months to years after the initial insult Damage to neurons Persistent inflammation and epigenetic changes Impaired neurogenesis Impaired axonal growth Altered synaptogenesis Decreased plasticity of the brain


Who is Eligible for Cooling

Cooling Eligibility Infants who are: >36 weeks (Some use 35 weeks) ≥ 1800 grams <6 hours old Meet inclusion criteria with blood gas or if blood gas not available has a history of an acute perinatal event and a 10 minutes APGAR ≤ 5 or assisted ventilation for 10 minutes And infant meets 3/6 criteria in Sarnat exam

ACOG Cord Gas Recommendations Low 5 minute Apgar (<5) Severe growth restriction/low birth weight Category III fetal heart rate pattern Intrapartum fever Also suggest: Meconium stained fluid, Breech delivery, multiple gestation, Cat. II FHR pattern, preterm delivery, abruptio placenta Simhan, H. , Barss, V. (2018). Umbilical cord blood acid-base analysis at delivery. In V. Barss (Ed. ), Upto. Date. Retrieved July 15, 2018, from https: //www. uptodate. com/contents/umbilical-cord-blood-acid-base-balance-at -deilvery.

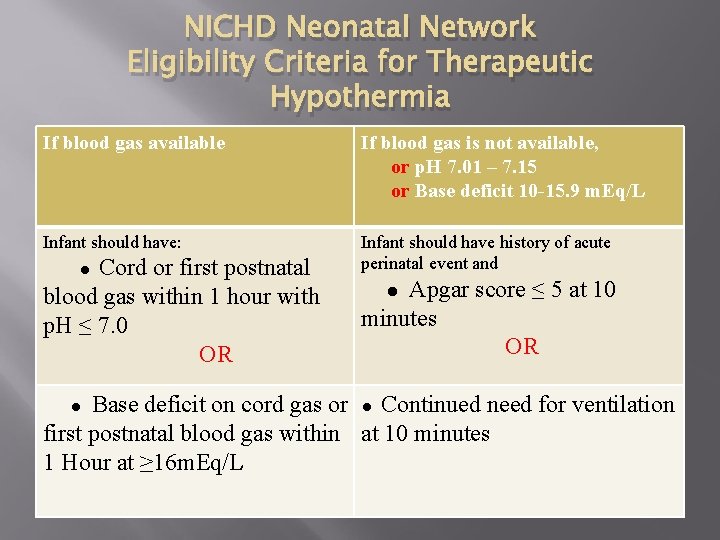
NICHD Neonatal Network Eligibility Criteria for Therapeutic Hypothermia If blood gas available If blood gas is not available, or p. H 7. 01 – 7. 15 or Base deficit 10 -15. 9 m. Eq/L Infant should have: Infant should have history of acute perinatal event and Cord or first postnatal blood gas within 1 hour with p. H ≤ 7. 0 OR ● Apgar score ≤ 5 at 10 minutes OR ● Base deficit on cord gas or ● Continued need for ventilation first postnatal blood gas within at 10 minutes 1 Hour at ≥ 16 m. Eq/L ●

Screening Algorithm and Criteria for Consultation California Perinatal Quality Care Collaborative ● 0 -10 minutes (Glass et al, Clin in Perin, 2016) Apgar ≤ 6 At 10 minutes ≥ 35 wks ≤ 6 hours Hx of acute perinatal event Continued PPV at 10 mins or CPR 10 -60 Minutes -Request cord blood gas or CBG -Obtain blood gas at <1 H life -Perform neuro exam -Observe for seizures Cord blood gas p. H ≤ 7 Or BE ≤-10

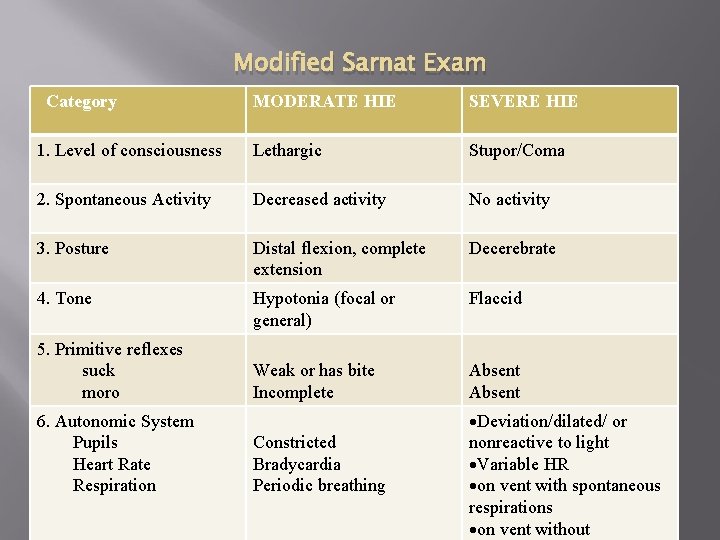
Modified Sarnat Exam Category MODERATE HIE SEVERE HIE 1. Level of consciousness Lethargic Stupor/Coma 2. Spontaneous Activity Decreased activity No activity 3. Posture Distal flexion, complete extension Decerebrate 4. Tone Hypotonia (focal or general) Flaccid Weak or has bite Incomplete Absent 5. Primitive reflexes suck moro 6. Autonomic System Pupils Heart Rate Respiration Constricted Bradycardia Periodic breathing Deviation/dilated/ or nonreactive to light Variable HR on vent with spontaneous respirations on vent without

The Modified Sarnat Exam Six categories (level of consciousness, spontaneous activity, posture, tone, primitive reflexes, and autonomic system) To be eligible for hypothermia: 3 of 6 categories have to be coded as either moderate or severe encephalopathy

Neurologic Exam Challenges Timing of exam Within first hour of life (before medications) May need to be performed by outlying provider Inter examiner variability Evolution of symptoms over first hours to days Examination findings may improve or get worse. Recommend serial exams at 1, 3, 5 hours Associated conditions: Respiratory distress, shoulder dystocia… Simultaneous mix of neurological findings Once you meet criteria you do not reassess with thought of recovery and not cooling the patient

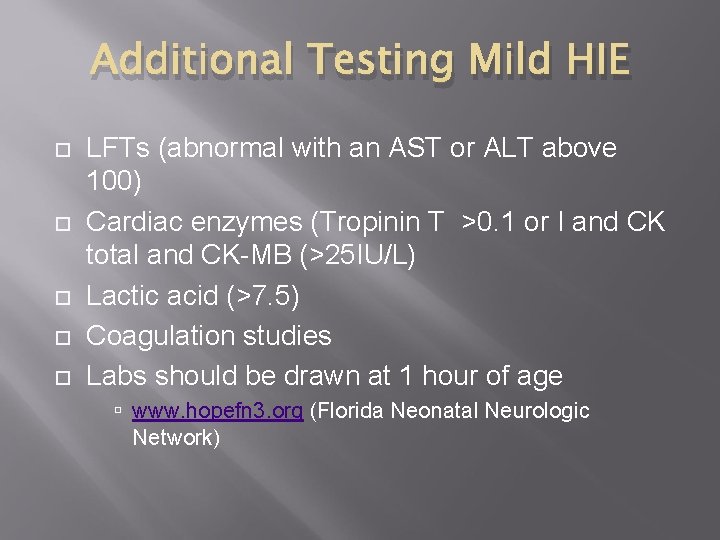
Additional Testing Mild HIE LFTs (abnormal with an AST or ALT above 100) Cardiac enzymes (Tropinin T >0. 1 or I and CK total and CK-MB (>25 IU/L) Lactic acid (>7. 5) Coagulation studies Labs should be drawn at 1 hour of age www. hopefn 3. org (Florida Neonatal Neurologic Network)

Decision Making in the Grey Zone

Who NOT to Cool Infants with traumatic or hemorrhagic brain and/or spinal cord injury but not evidence of asphyxia Infant’s with evidence of serious congenital CNS problem, Trisomy 13 or 18, microcephaly, syndrome with poor prognosis Parients who do no meet criteria for published randomized trial protocols Encephalopathy but no evidence for HI Infants < 35 weeks Infants >6 hrs old: efficacy questionable Infants in “extremis”. i. e. a reasonable neonatologist would not continue resuscitation or provide critical care

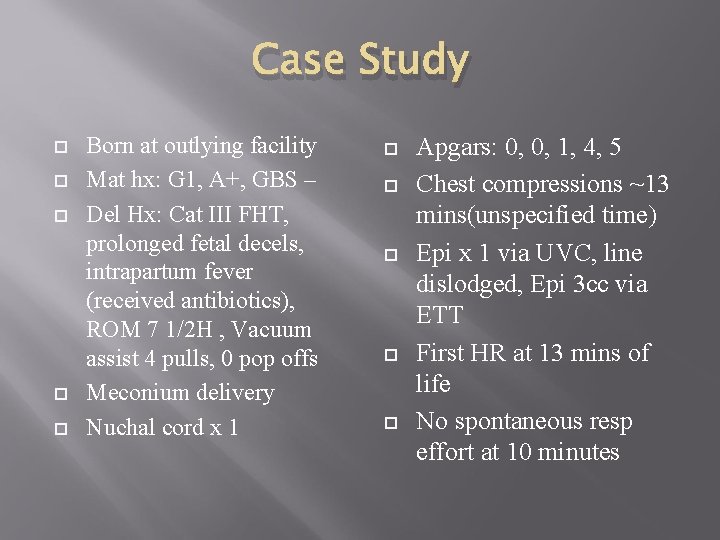
Case Study Born at outlying facility Mat hx: G 1, A+, GBS – Del Hx: Cat III FHT, prolonged fetal decels, intrapartum fever (received antibiotics), ROM 7 1/2 H , Vacuum assist 4 pulls, 0 pop offs Meconium delivery Nuchal cord x 1 Apgars: 0, 0, 1, 4, 5 Chest compressions ~13 mins(unspecified time) Epi x 1 via UVC, line dislodged, Epi 3 cc via ETT First HR at 13 mins of life No spontaneous resp effort at 10 minutes

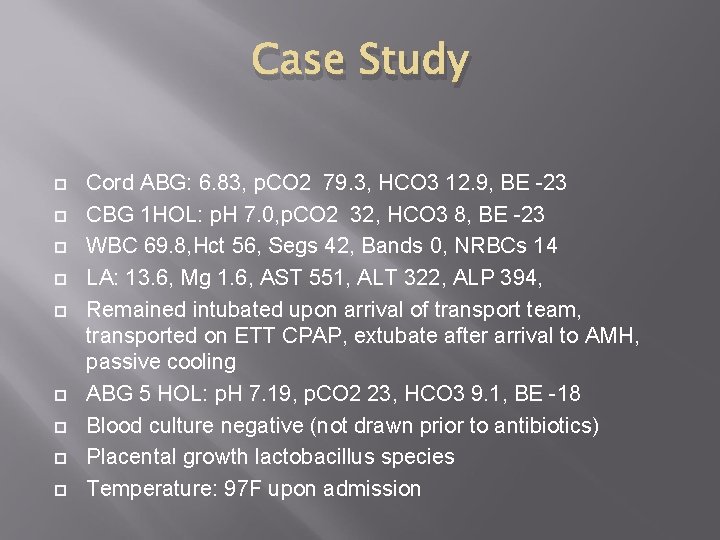
Case Study Cord ABG: 6. 83, p. CO 2 79. 3, HCO 3 12. 9, BE -23 CBG 1 HOL: p. H 7. 0, p. CO 2 32, HCO 3 8, BE -23 WBC 69. 8, Hct 56, Segs 42, Bands 0, NRBCs 14 LA: 13. 6, Mg 1. 6, AST 551, ALT 322, ALP 394, Remained intubated upon arrival of transport team, transported on ETT CPAP, extubate after arrival to AMH, passive cooling ABG 5 HOL: p. H 7. 19, p. CO 2 23, HCO 3 9. 1, BE -18 Blood culture negative (not drawn prior to antibiotics) Placental growth lactobacillus species Temperature: 97 F upon admission

Case Study c. EEG: suppressed background tracing without seizure activity DOL 5: Seizures, received Keppra and phenobarbital Last EEG showed intermittent, rhythmic focal slowing in both hemispheres MRI DOL 6: Diffuse profound hypoxic- ischemic brain injury with small subdural hemorrhage, restricted diffusion in the caudate nuclei, thalami, and cerebral cortex Prior to DC: Referred for hearing, no gag, g-tube dependent, minimal pupil reactivity, hypertonic lower extremities

Who NOT to Cool ? 3 Clinical Predictors of Severe Outcome Chest compressions for > 1 minute Onset of regular respirations > 30 minutes after birth Base deficit >16 on any blood gas within the first 4 hours of birth Severe outcome rates with none, two, or all 3 predictors were 46%, 64%, 76%, and 93% respectively. Wu, Y. (2017). Clinical features, diagnosis, and treatment of neonatal encephalopathy. In J. F. Dashe (Ed. ), Up to date. Retrieved October 21, 2017, from https: //www. uptodate. com/contents/neonatalencephalopathy

Poor Prognosis Indicators Lack of spontaneous respiratory effort within 20 -30 minutes of birth is almost always associated with death Presence of seizures Abnormal clinical neurologic findings persisting beyond the first 7 -10 days of life (hypotonia, rigidity, weakness) Persistent feeding difficulties, due to abnormal tone of oral muscles, suggest significant CNS damage. Poor head growth during postnatal period and first year of life

Poor Prognosis Indicators EEG that shows severe background abnormalities including burst suppression, isoelectricity or extremely low voltage portends and increased likelihood of death or significant long-term neurologic sequelae. In infants undergoing TH, persistently abnormal a. EEG at 48 hours of life is more predictive of adverse outcomes than an abnormal a. EEG at an earlier age.

Inborns vs Outborns

Outborn Infants Need more resuscitation Less likely to have cord gases Take longer to cool/longer to reach destination More likely to have more severe HIE/ brain injury on MRI Initiation of cooling at the birth hospital is often necessary with inexperienced personnel

Challenges with Transport for Cooling Time limited therapy (6 hours) Active/Passive cooling on transport may be necessary Sick babies with multisystem organ failure Unrecognized perinatal risk factors Safety Concerns: Patient safety: 10 -34% over-cooling in early passive cooling series Small studies report overcooling for infants with more severe asphyxia (O’Reilly, et al 2013) Vehicular safety issues Can cooling equipment be safely secured in transport system

Cooling Guidelines for Referring Hospitals Turn off radiant warmer Rectal temperature every 15 mins with a target temperature of 32. 5°C- ° 34 C R, 32°C-33°C Ax If temperature falls <33°C, turn on RW with temperature set 0. 5°C above current temperature, adjust q 15 mins until in desired range Call nearest hypothermia center for assistance (within two hours) Obtain blood gas within first hour. Perform neurologic exam immediately once stabilized (e. NICU) If in doubt, ship it out

Management Secure vascular access (PIV vs umbilical lines) Avoid hypoglycemia (glucose levels >50 mg/dl) Avoid hyperventilation and hyperoxygenation Target p. CO 2 = 40 -50 Target Pa. O 2 = 60 -100 mm. Hg, keep O 2 sats = 94 -98% Send blood cultures and consider IV antibiotics Consider additional lab work (Triponin, CK-MB, LFTs) Keep comfortable, minimize cold stress, avoid shivering. Indirect evidence for modest sedation improves neonatal outcomes Maintain adequate blood pressure and perfusion

Evidence for early initiation In animal models, cooling was most effective in reducing brain injury when started at 1. 5 hours following ischemia, was less effective at 5. 5 hours, and was not effective at 8. 5 hours (Gunn et al, Peds 1998). In the TOBY trial, infants treated within 4 hours of delivery benefited most from hypothermia therapy Ideally, the initial call to a regional cooling center is made within 2 hours of birth for all infants and in cases with more severe symptoms, call should be made within 1 hour of birth.

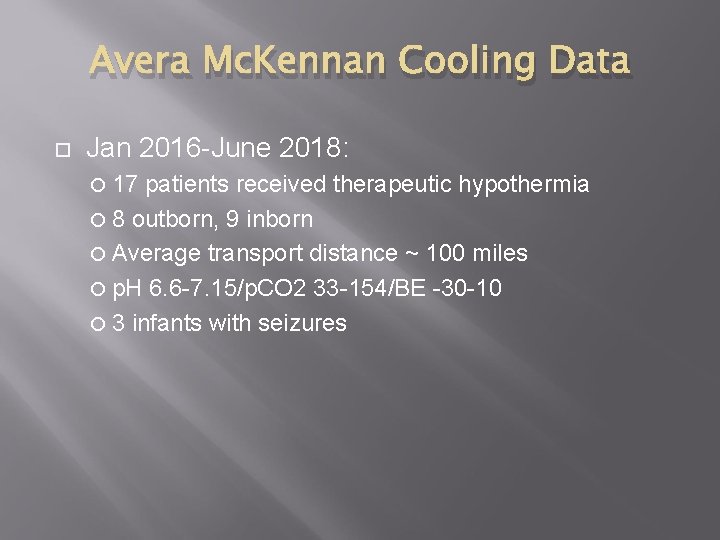
Avera Mc. Kennan Cooling Data Jan 2016 -June 2018: 17 patients received therapeutic hypothermia 8 outborn, 9 inborn Average transport distance ~ 100 miles p. H 6. 6 -7. 15/p. CO 2 33 -154/BE -30 -10 3 infants with seizures

How Well Does Cooling Work On average, cooling trials took (death or disability) from mid 60 s to upper 40 s. Number needed to treat : 8 to improve 1 Why doesn’t every baby benefit? Injury too old? Injury too severe? Treatment not maximally effective?

What About Mild Encephalpathy? Previously considered a benign clinical syndrome with a good long term prognosis Infants not considered eligible for cooling per current guidelines Outcomes less well studied Lack of definition Some newborns presenting with mild encephalopathy will develop more severe clinical or a. EEG findings over the first 48 hours of life

Treatment for Mild HIE? Many institutions develop individual enrollment criteria Increasing trend to provide TH to newborns with mild NE Encephalopathy is a dynamic process, neurologic status may worsen over 72 hours

Mild HIE…Are we Missing Something? Retrospective study, performed at single tertiary NICU 2013 -2015 89 infants received TH. 48 mild, 35 mod, 6 severe. Criteria: ↓GA ≥ 34 wks +1 of following criteria: p. H ≤ 7. 1 (7. 0), BE ≥-12 (-16), Apgar ≤ 5 at 10 mins, CC &/or PPV >10 mins of life NE graded by neonatologist + neurologist c. EEG 54% overall had abnormal MRI, no > difference between grade of NE Basal ganglia/thalamic injury more common in severe NE, watershed injury did not differ between grades Conclusion: Grade of NE during the first hours of life may not adequately discriminate between infants with and without cerebral injury Walsh et al. , J. Pediatrics, 2017

Mild HIE. . Abnormal ST Outcomes Retrospective study, single hospital, October 2005 -2008. All infants ≥ 36 wks, ≥ 1800 gm and had perinatal acidemia but did not qualify for TH based on neuro exam Screening criteria: 1)p. H ≤ 7. 0 or BE ≥-16 or 2)hx of acute perinatal event and either 1)no BG available or 2)p. H from 7. 01 -7. 15 or base deficit 10 -15. 9 m. Eq/L along with 10 min Apgar score ≤ 5 or PPV initiated at birth and continued for at least 10 minutes. If these criteria met, neurologic exam performed by certified neonatologist Medical records of infants who were deamed to have mild or no encephalopathy (did not receive TH) were reviewed for short term outcomes

Mild HIE…Abnormal Outcomes? Short term abnormal outcomes described as 1 of the following: Death ▫Abn neuro exam at DC Seizures ▫G-Tube feeding (unable to achieve full PO) Abn MRI 144/240 admission for perinatal acidemia met criteria for a neurologic exam. (96 excluded due to congenital anomolies, early death, transfer…) Age at exam not significantly different (29 normal exam, 60 mild NE, 48 mod HIE, 7 severe HIE). 89 infants form study population

Results of Study 89 infants with no or mild HIE had initial Sarnat exam performed 29 (33%) had absence of abnormalities on neuro exam 29 (33%) had 1 abnormal category 31 (35%) had 2 abnormal categories 60 infants described as having mild HIE 12 (20%) experienced an abnormal outcome 5 developed seizures between 12 and 40 hours of age (4/5) had abnormal MRIs with injury to watershed regions and basal ganglia , 5 th infant developed retractable seizures at 15 hol and died 2 hrs later from multi system organ failure 7 infants with abn neuro exam: hypotonia, hypertonia, facial palsy The highest PPV was achieved using autonomic system findings and 2+ abn categories with base deficit ≥ 15 within 1 hour of birth Du. Pont et al, Journal of Pediatrics, 2013, Jan. 162 (1), 35 -41.

Conclusions Grade of NE during the first hours of life may not adequately discriminate between infants with and without cerebral injury Questions regarding the reliability of the early neurologic exam Questions remain regarding the optimal timing of the exam Moderate encephalopathy most challenging yet is the stage with most potential for benefit

Can we Improve on Cooling Outcomes?

Use of Biomarkers and Imaging to Predict Outcomes Clinical presentation of HIE is varied, difficult to predict outcomes Use of biomarkers to help detect presence and severity of HIE Therapies could be targeted to maximum effect Chemicals measureable in blood, urine, CSF, or multiple locations to predict severity of brain injury Imaging performed in the first 6 hours of insult could help define severity of injury

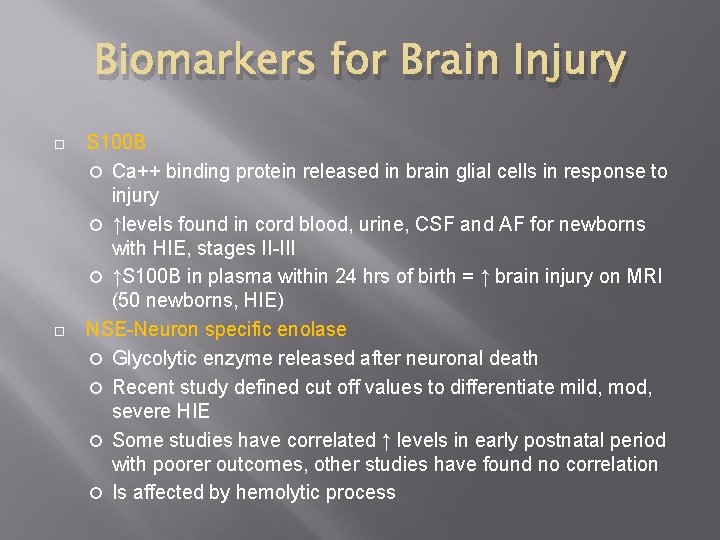
Biomarkers for Brain Injury S 100 B Ca++ binding protein released in brain glial cells in response to injury ↑levels found in cord blood, urine, CSF and AF for newborns with HIE, stages II-III ↑S 100 B in plasma within 24 hrs of birth = ↑ brain injury on MRI (50 newborns, HIE) NSE-Neuron specific enolase Glycolytic enzyme released after neuronal death Recent study defined cut off values to differentiate mild, mod, severe HIE Some studies have correlated ↑ levels in early postnatal period with poorer outcomes, other studies have found no correlation Is affected by hemolytic process

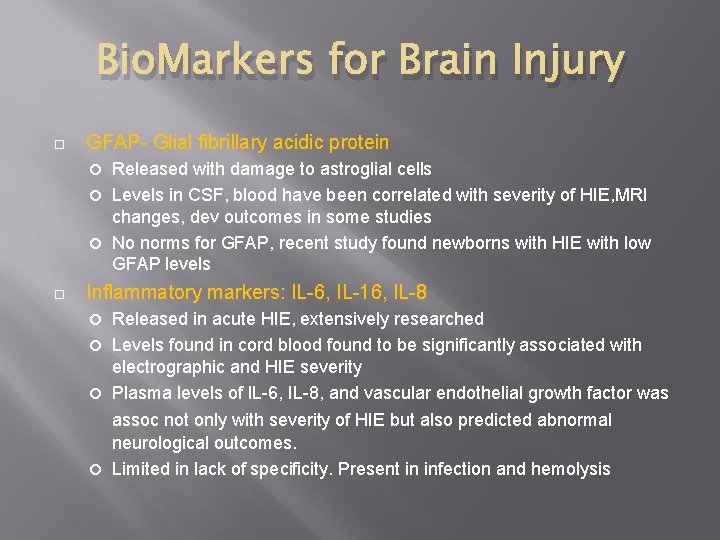
Bio. Markers for Brain Injury GFAP- Glial fibrillary acidic protein Released with damage to astroglial cells Levels in CSF, blood have been correlated with severity of HIE, MRI changes, dev outcomes in some studies No norms for GFAP, recent study found newborns with HIE with low GFAP levels Inflammatory markers: IL-6, IL-16, IL-8 Released in acute HIE, extensively researched Levels found in cord blood found to be significantly associated with electrographic and HIE severity Plasma levels of IL-6, IL-8, and vascular endothelial growth factor was assoc not only with severity of HIE but also predicted abnormal neurological outcomes. Limited in lack of specificity. Present in infection and hemolysis

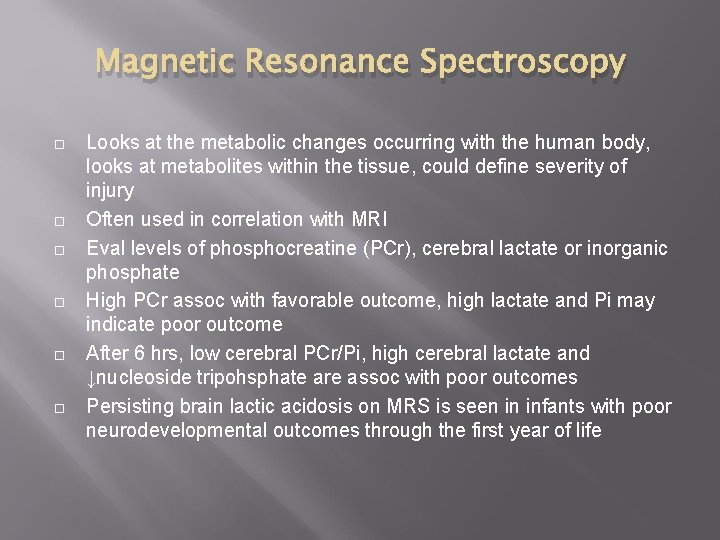
Magnetic Resonance Spectroscopy Looks at the metabolic changes occurring with the human body, looks at metabolites within the tissue, could define severity of injury Often used in correlation with MRI Eval levels of phosphocreatine (PCr), cerebral lactate or inorganic phosphate High PCr assoc with favorable outcome, high lactate and Pi may indicate poor outcome After 6 hrs, low cerebral PCr/Pi, high cerebral lactate and ↓nucleoside tripohsphate are assoc with poor outcomes Persisting brain lactic acidosis on MRS is seen in infants with poor neurodevelopmental outcomes through the first year of life

Is More Cooling Better? The NICHD Neonatal Research Network (NRN) “Optimizing Cooling Trial” Factorial design: 4 groups (1 = “conventional cooling”) 33. 5 vs 32°C 72 vs 120 hours Planned enrollment 726 Study terminated Nov. 27, 2013 by NICHD Director based on DSMC recommendation, for futility and safety concerns (364 enrolled) Preliminary findings: JAMA 312: 2629 -39, 2014 Primary outcome: JAMA 318: 57 -67, 2017

Optimizing Cooling Conclusions: Futility analysis determined that the probability of detecting a statistically significant benefit for longer cooling, deeper cooling, or both for NICU death was less than 2%. Safety outcomes were similar between the 120 hours group vs 72 hours group and the 32°C group vs. 33. 5°C group, except major bleeding occurred among 1% in the 120 hours group vs 3% in the 72 hours group (RR, 0. 25 {95% CI, 0. 07 -0. 91}).

Optimizing Cooling: 18 month Outcome Longer or deeper cooling neither decreased mortality nor decreased disability Adjusted relative risks for combined outcome were not different by duration or depth Rate of 1° outcome in standard care group lower than expected: 29% (vs 44% in 2005 NEJM paper) Baseline severity did not impact conclusion However: Early termination – reduced statistical power Shankaran et al. , JAMA 318: 57 -67, 2017

Late Therapeutic Hypothermia What if we miss the 6 hour window? NICHD NRN-NCT 00614744: Late Hypothermia Trial Enrolled 168 infants with HIE to receive hypothermia (96 h 33. 5° )vs. usual care presenting 6 -24 hours after birth. Primary outcome: Death or mod-severe disability at 18 mos adjusted for severity of encephalopathy and age at randomization Bayesian analysis –”statistical inference allowing one to combine prior information with evidence collected to guide statistical inference”

Late Therapeutic Hypothermia 76% probability of at least a 1% reduction in death or disability at 18 months 64% probability of at least 2% less death or disability No increased risk of serious adverse effects r/t TH Earlier cooling remains critical in outcomes but some may be better than none Laptook et al. , JAMA 318: 1550 -1560, 2017

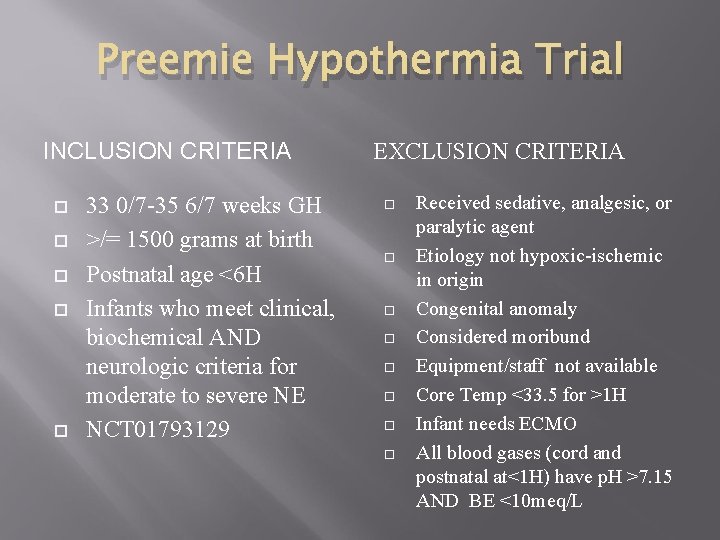
Preemie Hypothermia Trial INCLUSION CRITERIA 33 0/7 -35 6/7 weeks GH >/= 1500 grams at birth Postnatal age <6 H Infants who meet clinical, biochemical AND neurologic criteria for moderate to severe NE NCT 01793129 EXCLUSION CRITERIA Received sedative, analgesic, or paralytic agent Etiology not hypoxic-ischemic in origin Congenital anomaly Considered moribund Equipment/staff not available Core Temp <33. 5 for >1 H Infant needs ECMO All blood gases (cord and postnatal at<1 H) have p. H >7. 15 AND BE <10 meq/L

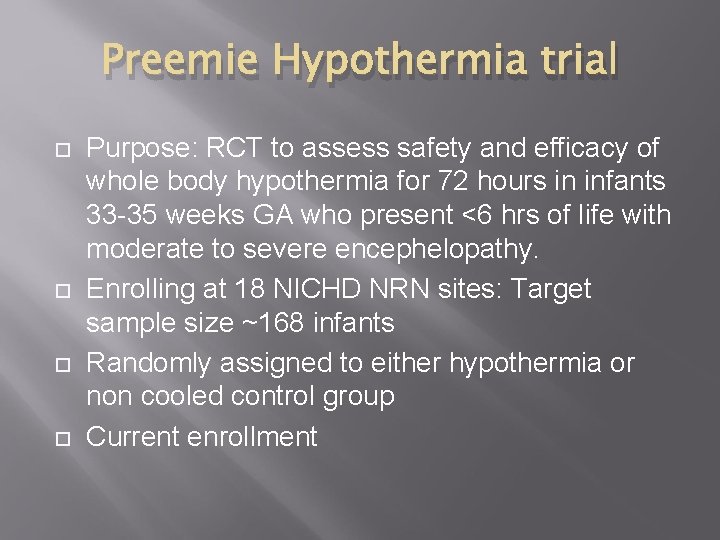
Preemie Hypothermia trial Purpose: RCT to assess safety and efficacy of whole body hypothermia for 72 hours in infants 33 -35 weeks GA who present <6 hrs of life with moderate to severe encephelopathy. Enrolling at 18 NICHD NRN sites: Target sample size ~168 infants Randomly assigned to either hypothermia or non cooled control group Current enrollment

Outcomes Primary: Death or moderate to severe disability at 18 -22 months corrected age As determined by standard NRN interdisciplinary follow up exam Secondary study: Determining an association between MRI detectable injury and neurodevelopment at 18 -22 month.

Adjunct Therapies to Therapeutic Hypothermia TH standard of care but has not definitively changed outcomes in severe HIE TH continues with limitations for use. Accessibility Financial Timing Possible use in mild encephalopathy We’re doing good…. can we do better?

Early Postnatal Pharmacologic Strategies Allopurinol ▫Xanthine oxidase inhibitor, ↓production of O 2 radicals ▫ Given to pregnant women just before delivery dx with fetal hypoxia ▫Short term outcomes-gender specific (female), vast majority of hypoxia was mild ▫Few , small human trials have shown neuroprotective effect given immediately after birth ▫Recruiting in Belgium, Austria Xenon ▪ NMDA /ion channel antagonist. Inhaled via ETT of 50% xenon for 18 hours within 5 hours of birth Not relevant in US- not FDA approved No US trials. Currently recruiting in United Kingdom Expensive, need cuffed tubes

Adjunct Pharmacological Therapies Melatonin Anti-apoptotic, anti-inflammatory, anti-oxidant effects Piglet models showed decreased HI –induced injury measured by MRS. ↓seizures, white matter injury, and mortality without neurologic abnormalities Good safety profile, low risk for toxicity, no consensus on optimal dose Currently recruiting for dosing studies, Florida NCT 02621944 Azithromycin Preclinical studies in models of ischemic stroke revealed neuroprotective effects Established safety profile. No studies to date

Adjunct Pharmacological Therapies Magnesium Sulfate NMDA receptor antagonist, ↓excitotoxic damage Initial interest generated due to noted low Mg levels in asphyxiated infants Meta analysis of 5 RCTs showed positive short term outcomes Concerns re: hypotension and bradycardia Current studies performed of Mg combined with moderate hypothermia (Malaysia) Topiramate Anticonvulsant agent Current study hypothesis: adjunct therapy will reduce short term severity of HIE, seizures, reduce normalization time of the a. EEG. Infants will receive 5 mg/kg of drug daily enterally x 5 doses UC Davis Medical Center NCT 01765218

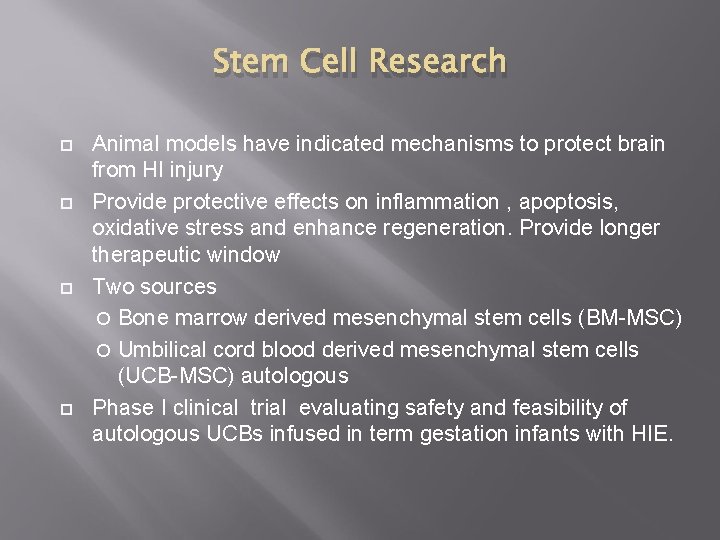
Stem Cell Research Animal models have indicated mechanisms to protect brain from HI injury Provide protective effects on inflammation , apoptosis, oxidative stress and enhance regeneration. Provide longer therapeutic window Two sources Bone marrow derived mesenchymal stem cells (BM-MSC) Umbilical cord blood derived mesenchymal stem cells (UCB-MSC) autologous Phase I clinical trial evaluating safety and feasibility of autologous UCBs infused in term gestation infants with HIE.

Baby. BAC II Trial A phase II multi-site study of autologous cord blood cells for HIE Purpose: Assess safety and efficacy of up to two intravenous infusion of volume reduced autologous umbilical cord blood cells compared to placebo in neonates with HIE undergoing standard of care TH. Determine if providing cord blood to infants with HIE improves outcomes as measured at 12 months of age through both neurological and developmental testing Goal: Enroll 160 subjects

Baby. Bac II Trial INCLUSION CRITERIA >36 weeks GA who qualify for cooling Cord Blood Available for volume and red blood cell reduction before 45 hours of age Newborn must be able to receive autologous cord blood or placebo before 48 H of age EXCLUSION CRITERIA Non viable infant Cong. Chrom anomalies Severe IUGR(<1800 g) Mother with HIV, Hep BC, syphy, Zika, CMV Sepsis/Chorioamnionitis Study interferes with safety of infant or treatment

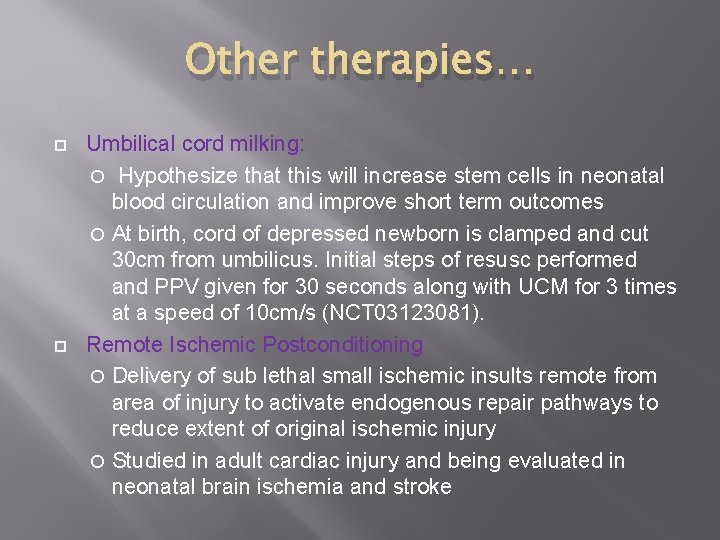
Otherapies… Umbilical cord milking: Hypothesize that this will increase stem cells in neonatal blood circulation and improve short term outcomes At birth, cord of depressed newborn is clamped and cut 30 cm from umbilicus. Initial steps of resusc performed and PPV given for 30 seconds along with UCM for 3 times at a speed of 10 cm/s (NCT 03123081). Remote Ischemic Postconditioning Delivery of sub lethal small ischemic insults remote from area of injury to activate endogenous repair pathways to reduce extent of original ischemic injury Studied in adult cardiac injury and being evaluated in neonatal brain ischemia and stroke

Erythropoietin Therapy Most attractive candidate Anti-oxidant and anti-inflammatory effect Shown to improve neurodevelopmental outcomes in studies of preterm infants Phase I/II trials (NEAT /NEATO trial) 2012 Safety and pharmacokinetics study of high doses of EPO to newborn infants with asphyxia Combined these trials suggested that administering five doses (1000 u/kg) over the baby’s first week of life was safe and possibly effective. NEATO trial used MRI of the neonate’s brain and 1 year behavioral outcomes to document its results. Infant’s shown less brain injury on early MRI and better 1 yr outcomes. Findings have led to Phase III trial (HEAL) and (PEAEN)

HEAL Trial High-Dose Epo for Asphyxia and Encephalopathy Hypothesis High dose given to cooled infants with moderate/severe HIE will reduce the primary outcome of death or NDI at 22 -26 month from 49% to 33% Epo administration will be safe, will decrease brain injury severity on MRI and will decrease serial inflammatory cytokines and biomarkers of brain injury Specific aims are to determine if 5 doses of Epo 1000 u/kg will reduce rate death, cognitive, or motor deficits at 2 year. Assess safety of Epo by evaluating clinical toxicity Determine if Epo decreases the severity of brain injury as determined by early biomarkers Recruiting 500 infants (HEAL) in US

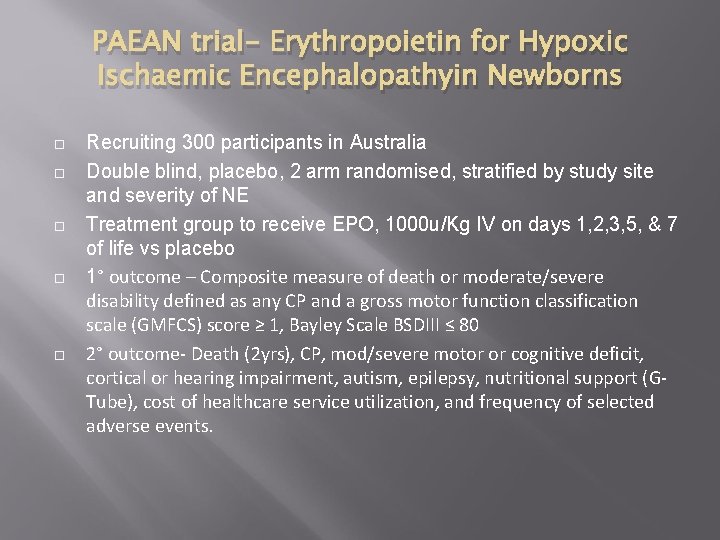
PAEAN trial- Erythropoietin for Hypoxic Ischaemic Encephalopathyin Newborns Recruiting 300 participants in Australia Double blind, placebo, 2 arm randomised, stratified by study site and severity of NE Treatment group to receive EPO, 1000 u/Kg IV on days 1, 2, 3, 5, & 7 of life vs placebo 1° outcome – Composite measure of death or moderate/severe disability defined as any CP and a gross motor function classification scale (GMFCS) score ≥ 1, Bayley Scale BSDIII ≤ 80 2° outcome- Death (2 yrs), CP, mod/severe motor or cognitive deficit, cortical or hearing impairment, autism, epilepsy, nutritional support (GTube), cost of healthcare service utilization, and frequency of selected adverse events.

Darbepoietin Administration in Newborns Undergoing Cooling for Encephalopathy (DANCE) Phase I/II trials, N=30, 10/group. Receive Darbe 2 or 10 mcg/Kg (placebo) within 12 H of birth and at 7 d. 1°- Evaluating safety of Darbe when used as an adjunctive therapy to hypothermia 2°-Participants with adverse events: eg. . BP, infection, neutropenia, thrombolytic, hematologic and hepatic/renal function Conclusion: Darbe combined with hypothermia has similar safety profile to placebo with pharmacokinetics sufficient for weekly administration Baserga, M. C. et al Pediatric Res. 2015 Sep: 78 (3): 315 -322. Phase II trial NCT 03071861 Mild Encephalopathy in the Newborn Treated with Darbepoetin (MEND). Currently recruiting Infants who do not meet clinical criteria for TH

What Can We Do? Provide early and rapid recognition of infant’s eligible for cooling Perform early cooling as best as possible, may need to be initiated at hospital of birth Provide for quick identification and transfer of infant’s eligible Consult with cooling center if on the fence regarding infant’s eligibility Continue to collect, provide data to national database

References Barks, J. (2018, May). Update on diagnosis and management of hypoxic-ischemic encephalopathy (HIE) in 2018. Presented at Academy of Neonatal Nursing, Portland, Oregon. Bel, F. , and Groenendaal, F. (2016). Drugs for neuroprotection after birth asphyxia: Pharmacological adjuncts to hypothermia. Seminars in Perinatology, 40, 152 -159. Du. Pont, T. , Chalak, L. , Morriss, M. , Burchfield, P. J. , Christie, L. , and Sanchez, P. (2013). Short-term outcomes of newborns with perinatal acidemia who are not eligible for systemic hypothermia therapy. Journal of Pediatrics, 162 (1), 35 -41. Ergenekon, E. , (2016). Therapeutic hypothermia in neonatal intensive care unit: Challenges and practical points. Journal In Clinical Neonatology, 5, 8 -17. Gagne-Loranger, M. , Sheppard, M. , Ali, N. , Saint-Martin, C. , and Wintermark, P. (2015). Newborns referred for therapeutic hypothermia: Association between initial degree of encephalopathyand severity of bran injury (what about the newborns with mild encephalopahty on admission? ). American Journal of Perinatology, 33, 195 -202. Glass, H. and Rowitch, D. (2016) The role of the neurointensive care nursery for neonatal encephalopathy. Clinics in Perinatology, 43, 547 -557. Karnatovskaia, L. , Wartenberg, K. , and Freeman, W. (2014). Therapeutic hypothermia for neuroprotection. Neurohospitalist, 4 (3), 153 -163. Laptook, AR. , Shankaran, S. , Tyson, JE. , Munoz, B. , Bell, EF. , and Goldberg, RN. (2017). Does late therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic ischemic encephalopathy: A randomized clinical trial. JAMA, 318, 1550 -1560.

References Millar, L. , Shi, L. , Hoerder-Suabedissen, A. , and Molnar, Z. (2017). Neonatal hypoxia ischaemia: Mechanisms, models and therapeutic Challenges. Front Cell Neuroscience, 11: 78. Olson, S. , Dejong, M. , Kline, Al, Liptseen, El, Song, D. , Anderson, B. , and Mathur, A. (2013). Optimizing therapeutic hypothermia for neonatal encephalopathy. 131 (2). Retrieved from http: //www. pediatrics. aappublications. org. O’Reilly, D. , Labrecque, M. , O’Melia, M. , Bacic, J. , Hansen, A. , and Soul, J. (2013). Passive cooling during transport of asphyxiated newborns. Journal of Perinatology, 33 (6), 435 -440. Rao, R. , Trivedi, S. , Vesoulis, Z. , Liao, S. , Smyser, C. , and Mathur, A. (2017). Safety and short term outcomes of therapeutic hypothermia in preterm neonates 34 -35 weeks gestational age with hypoxic-ischemic encephalopathy. The Journal of Pediatrics, 183. 37 -42. Schierholz, E. (2014). Therapeutic hypothermia on transport. Providing safe and effective cooling therapy as the link between birth hospital and the neonatal intensive care unit. Advances in Neonatal Care, 14 (5 s), S 24 -S 31. Thoresen, M. (2008). Supportive care during neuroprotective hypothermia in the term newborn: Adverse effects and their prevention. Clinics in Perinatology, 35, 749 -763.

References Van. Bel, F. , and Groenendaal, F. (2016). Drugs for neuroprotection after birth asphyxia: Pharmacologic adjuncts to hypothermia. Seminars in Perinatology, 40, 152 -159. Wu, U. (2017). Clinical features, diagnosis, and treatment of neonatal encephalopathy. In J. F. Dashe (Ed. ), Up. To. Date. Retrieved October 21, 2017, from https: //www. uptodate. com/contents/neonatalencephalopathy
 Shadow paging recovery technique
Shadow paging recovery technique Chilblains vs trench foot
Chilblains vs trench foot Temperature hypothermia
Temperature hypothermia Hypothermia suffix
Hypothermia suffix 5 phases of the nursing process
5 phases of the nursing process Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Nursing diagnosis three parts
Nursing diagnosis three parts Nursing process objectives
Nursing process objectives Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Hie grading
Hie grading Open hie
Open hie Open hie
Open hie Simone hie
Simone hie Roshini jayasankar
Roshini jayasankar Open hie
Open hie Apgar
Apgar Iap slides
Iap slides Hie transistor
Hie transistor Camden hie
Camden hie Open hie
Open hie Open hie
Open hie Kansas hie
Kansas hie Alaska hie
Alaska hie Simone hié
Simone hié Introduction of milieu therapy
Introduction of milieu therapy Therapeutic class and pharmacologic class
Therapeutic class and pharmacologic class Association for applied and therapeutic humor
Association for applied and therapeutic humor Basic knowledge of food and beverage
Basic knowledge of food and beverage Therapeutic orientations
Therapeutic orientations Therapeutic feeding center adalah
Therapeutic feeding center adalah Therapeutic exercise chapter 1 mcqs
Therapeutic exercise chapter 1 mcqs Microwave diathermy block diagram
Microwave diathermy block diagram Therapeutic drift
Therapeutic drift Therapeutic communication introduction
Therapeutic communication introduction What is the triangle of care
What is the triangle of care Therapeutic story writing starters
Therapeutic story writing starters Component of therapeutic communication
Component of therapeutic communication Therapeutic exercise prescription
Therapeutic exercise prescription Efficacy definition pharmacology
Efficacy definition pharmacology High therapeutic index
High therapeutic index Therapeutic index
Therapeutic index Classification of therapeutic exercise
Classification of therapeutic exercise Be intentional in relationships
Be intentional in relationships 5 healthcare pathways
5 healthcare pathways Reproductive cloning process
Reproductive cloning process Multicultural therapeutic communication skills
Multicultural therapeutic communication skills Transport technician healthcare pathway
Transport technician healthcare pathway Therapeutic index
Therapeutic index Therapeutic substitution vs interchange
Therapeutic substitution vs interchange Therapeutic environment in hospital
Therapeutic environment in hospital Therapeutic value of play
Therapeutic value of play Non therapeutic
Non therapeutic Competitive and non competitive antagonist
Competitive and non competitive antagonist What is habilitative exercise?
What is habilitative exercise? Therapeutic riding lesson plan examples
Therapeutic riding lesson plan examples Therapeutic drift
Therapeutic drift Therapeutic communication definition
Therapeutic communication definition Therapeutic window
Therapeutic window Ichaemic
Ichaemic Chapter 4 therapeutic communication skills
Chapter 4 therapeutic communication skills Therapeutic recreation program sydney
Therapeutic recreation program sydney Interim therapeutic restoration
Interim therapeutic restoration Btpb repertory
Btpb repertory Therapeutic window
Therapeutic window Therapeutic window
Therapeutic window Therapeutic touch
Therapeutic touch Therapeutic photography exercises
Therapeutic photography exercises Full liquid diet definition
Full liquid diet definition Pre-hospital communication
Pre-hospital communication Incompatibilities in pharmaceutical dosage forms
Incompatibilities in pharmaceutical dosage forms Examples of therapeutic services
Examples of therapeutic services Angela wadham steps
Angela wadham steps Therapeutic communication techniques
Therapeutic communication techniques Therapeutic recreation models
Therapeutic recreation models Therapeutic interview
Therapeutic interview Faradic current
Faradic current Therapeutic dialogue
Therapeutic dialogue Therapeutic window
Therapeutic window Therapeutic exercise definition
Therapeutic exercise definition