Theory of Dyeing and Experiment Fashion Industry School
























![Diazo화 과정 Ar-NH 2 Na. NO 2/HCl diazo화 Ar-N 2+]Cl- Ar-N=N-Ar’ azo염료 Ar’-H coupling성분 Diazo화 과정 Ar-NH 2 Na. NO 2/HCl diazo화 Ar-N 2+]Cl- Ar-N=N-Ar’ azo염료 Ar’-H coupling성분](https://slidetodoc.com/presentation_image_h2/9b308ec72d912dfd1bc5e4cf20ebcfae/image-55.jpg)





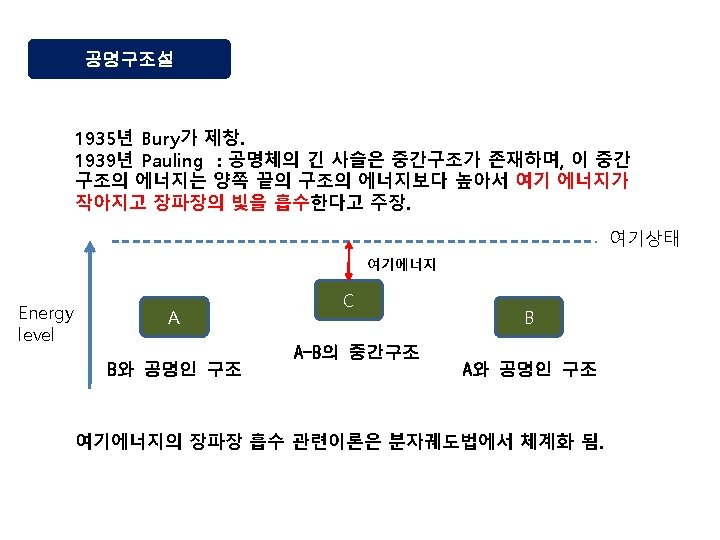
- Slides: 72

염색이론 및 실험 Theory of Dyeing and Experiment 패션산업학부 Fashion Industry School

염료이론 Theory of Dyestuff














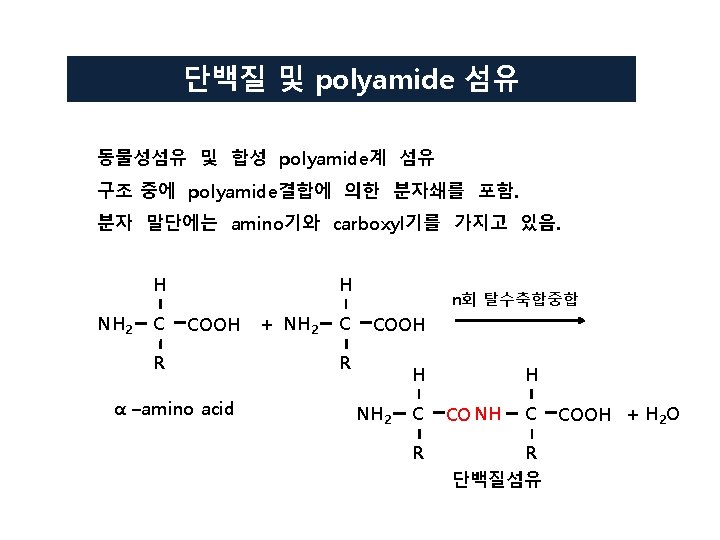
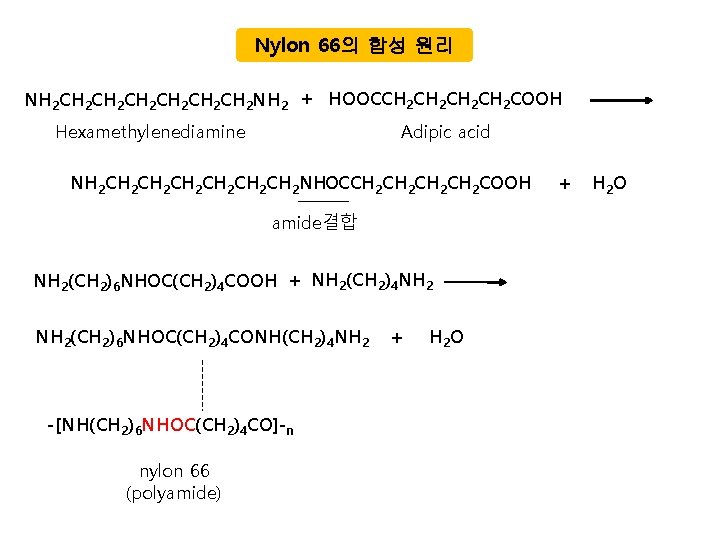
Nylon 66의 합성 원리 NH 2 CH 2 CH 2 CH 2 NH 2 + HOOCCH 2 CH 2 COOH Hexamethylenediamine Adipic acid NH 2 CH 2 CH 2 CH 2 NHOCCH 2 CH 2 COOH amide결합 NH 2(CH 2)6 NHOC(CH 2)4 COOH + NH 2(CH 2)4 NH 2(CH 2)6 NHOC(CH 2)4 CONH(CH 2)4 NH 2 -[NH(CH 2)6 NHOC(CH 2)4 CO]-n nylon 66 (polyamide) + H 2 O


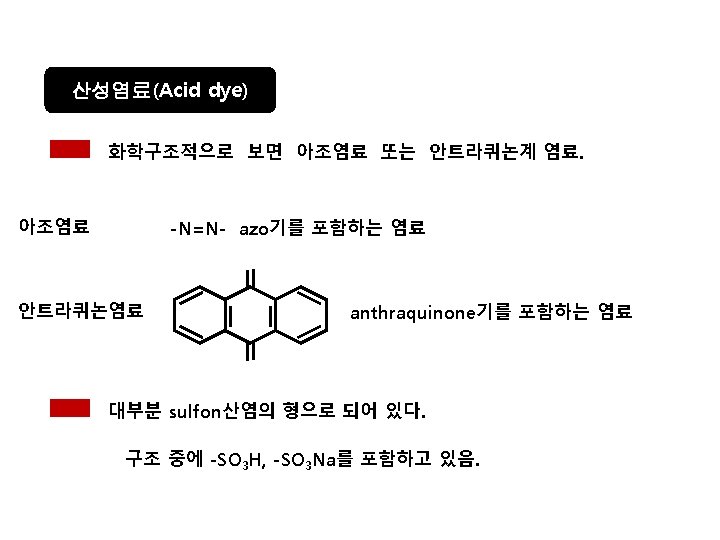
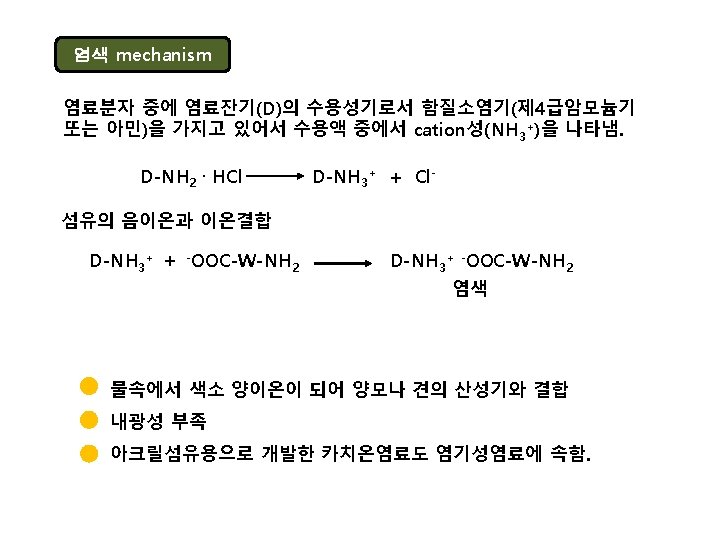
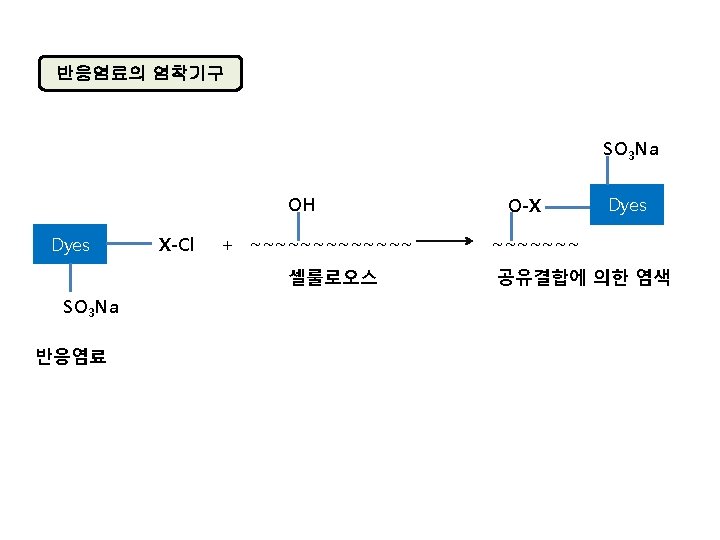
Acid dye An acid dye is a dye which is a salt of a sulfuric, carboxylic or phenolic organic acid. The salts are often sodium or ammonium salts. Acid dyes are typically soluble in water and possesses affinity for amphoteric fibers while lacking direct dyes' affinity for cellulose fibers. When dyeing, ionic bonding with fiber cationic sites accounts for fixation of colored anions in the dyed material. Acids are added to dyeing baths to increase the number of protonated amino-groups in fibers.

amphoteric When you think of amphoteric substances, think of a dualpurpose product that we can buy in the store, like, for example, two-in-one shampoo and conditioner. This product can clean our hair and condition and soften our hair at the same time. The same dual-action goes for amphoteric substances. The prefix of the word 'amphoteric' is derived from a Greek prefix amphi-, which means both. In chemistry, an amphoteric substance is a substance that has the ability to act either as an acid or a base. Remember that acids donate protons (or accept electron pairs), and bases accept protons. Amphoteric substances can do either. So, we can think of an amphoteric substance as something like a double agent.

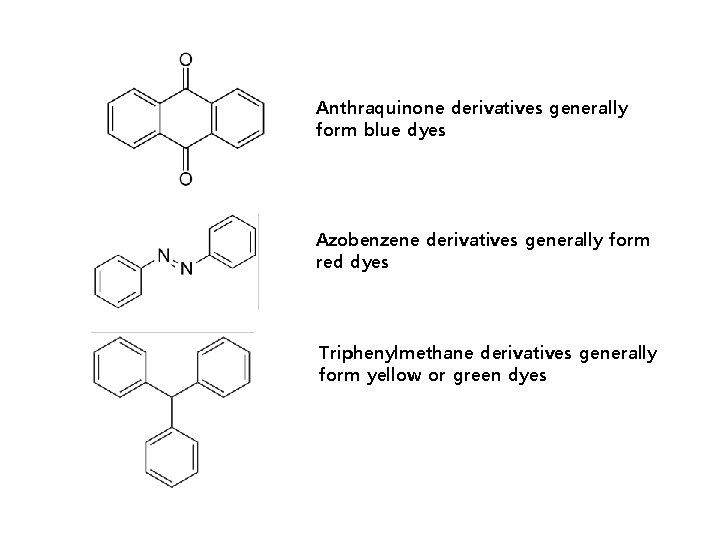
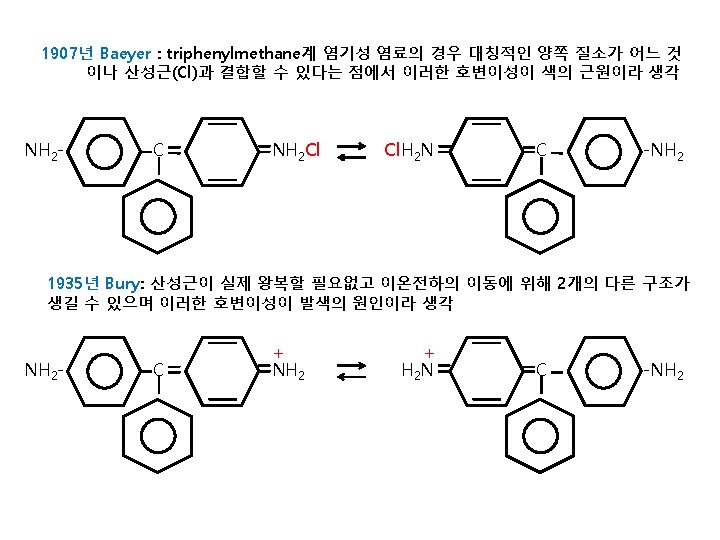
Structures of Acid dyes The chemistry of acid dyes is quite complex. Dyes are normally very large aromatic molecules consisting of many linked rings. Acid dyes usually have a sulfo or carboxy group on the molecule making them soluble in water. Water is the medium in which dyeing takes place. Most acid dyes are related in basic structure to the following: Anthraquinone type: Many acid dyes are synthesized from chemical intermediates which form anthraquinone-like structures as their final state. Many blue dyes have this structure as their basic shape. The structure predominates in the levelling class of acid dye. Azo dyes: The structure of azo dyes is based on azobenzene, Ph-N=N−Ph (see image on right showing cis/trans isomers). Although azo dyes are a separate class of dyestuff mainly used in the dyeing of cotton (cellulose) fibers, many acid dyes have a similar structure, and most are red in color. Triphenylmethane related: Acid dyes having structures related to triphenylmethane predominate in the milling class of dye. There are many yellow and green dyes commercially applied to fibers that are related to triphenylmethane.

Anthraquinone derivatives generally form blue dyes Azobenzene derivatives generally form red dyes Triphenylmethane derivatives generally form yellow or green dyes






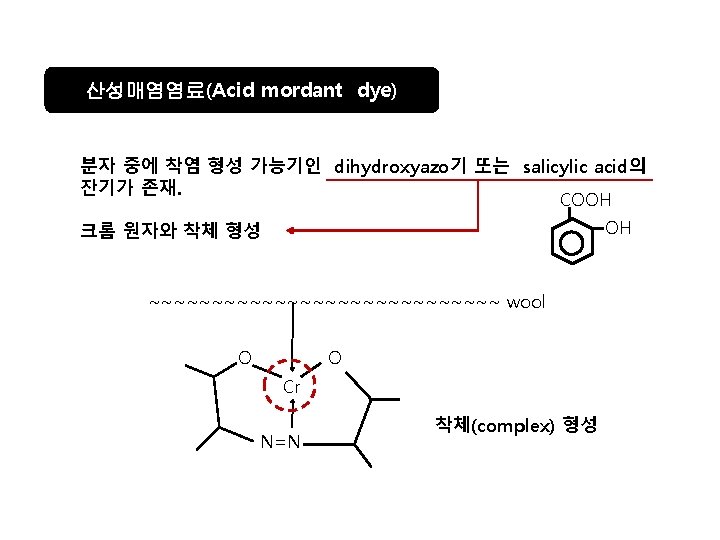
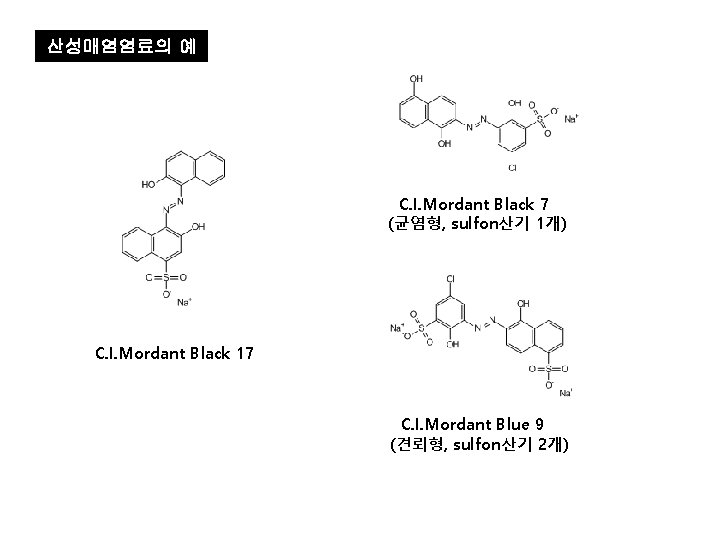
산성매염염료의 예 C. I. Mordant Black 7 (균염형, sulfon산기 1개) C. I. Mordant Black 17 C. I. Mordant Blue 9 (견뢰형, sulfon산기 2개)


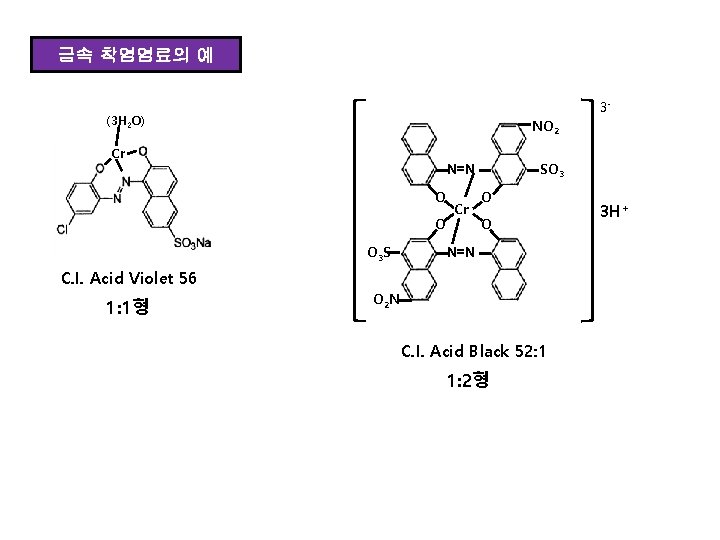
금속 착염염료의 예 3 - (3 H 2 O) NO 2 Cr SO 3 N=N O O O 3 S Cr O O N=N C. I. Acid Violet 56 1: 1형 O 2 N C. I. Acid Black 52: 1 1: 2형 3 H+

염기성염료(Basic dye) Basic dyes are water-soluble cationic dyes that are mainly applied to acrylic fibers, but find some use for wool and silk. Usually acetic acid is added to the dyebath to help the uptake of the dye onto the fiber. Basic dyes are also used in the coloration of paper. 구형 염기성염료 1800년대 후반~1900년대 초. 주로 목면의 매염염색용을 개발된 것 직접염료, 건염염료, 반응염료에 의해 밀려남. 현재는 종이, 펄프, 피혁, 잡화류 염색, 잉크, lake등에 이용 카치온염료 1930년대 이후 아세테이트, 아크릴, 염기성염료 가염형 합성염료 용으로 개발한 것


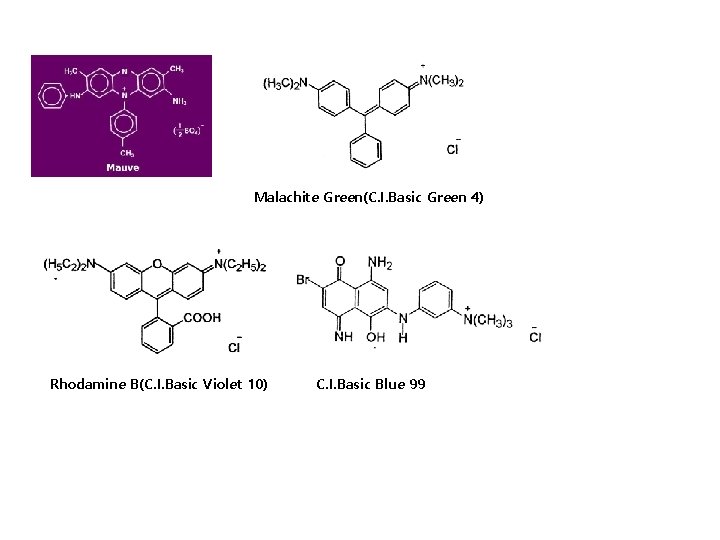
Malachite Green(C. I. Basic Green 4) Rhodamine B(C. I. Basic Violet 10) C. I. Basic Blue 99


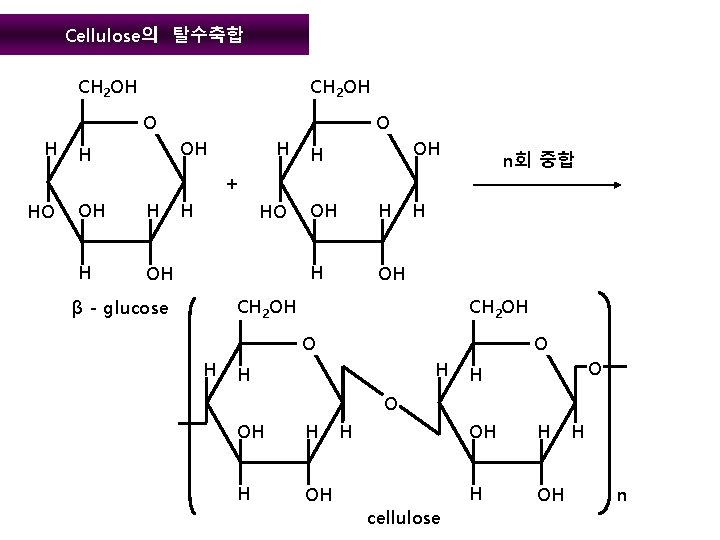
Cellulose의 탈수축합 CH 2 OH O H O OH H H OH H n회 중합 + HO OH H H OH H CH 2 OH β - glucose CH 2 OH O H H O OH H H OH H cellulose OH H H OH H n












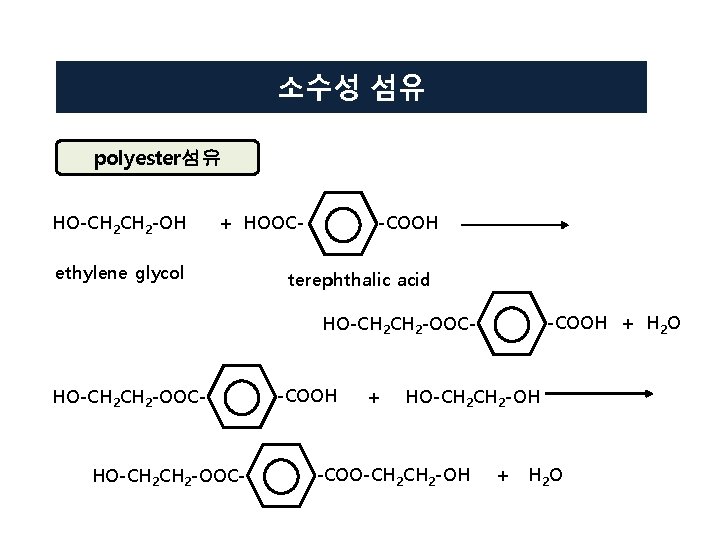
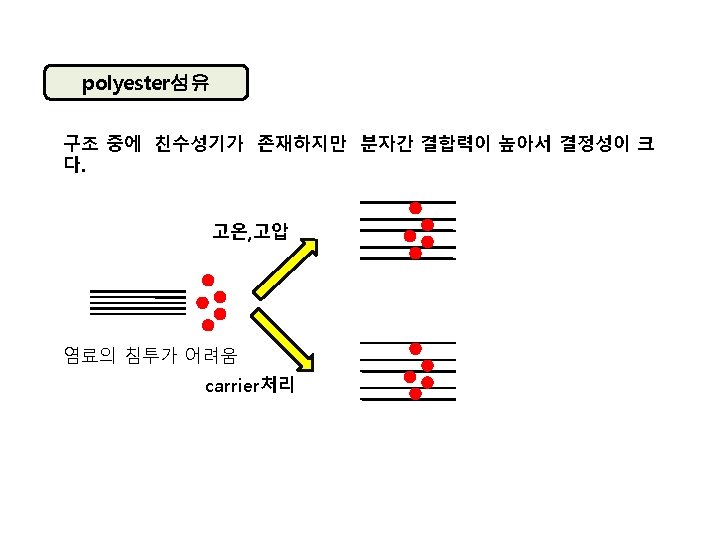
소수성 섬유 polyester섬유 HO-CH 2 -OH + HOOC- ethylene glycol -COOH terephthalic acid -COOH + H 2 O HO-CH 2 CH 2 -OOC- HO-CH 2 -OOC- -COOH + HO-CH 2 -OH -COO-CH 2 -OH + H 2 O






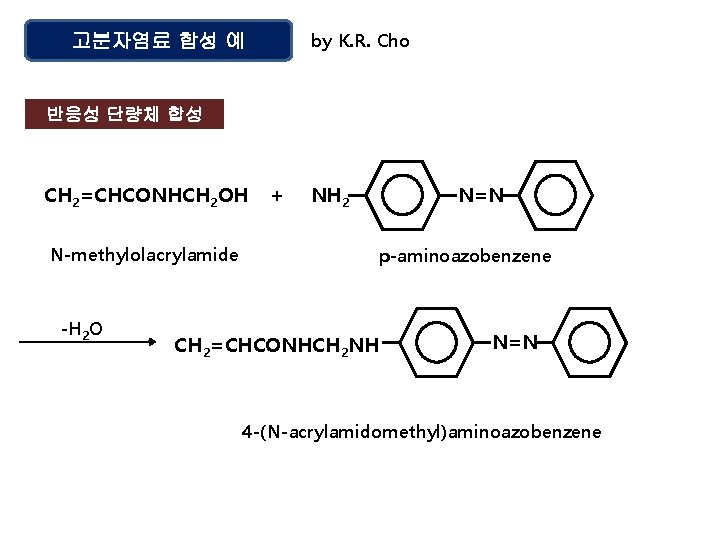
by K. R. Cho 고분자염료 합성 예 반응성 단량체 합성 CH 2=CHCONHCH 2 OH N-methylolacrylamide -H 2 O + NH 2 N=N p-aminoazobenzene CH 2=CHCONHCH 2 NH N=N 4 -(N-acrylamidomethyl)aminoazobenzene

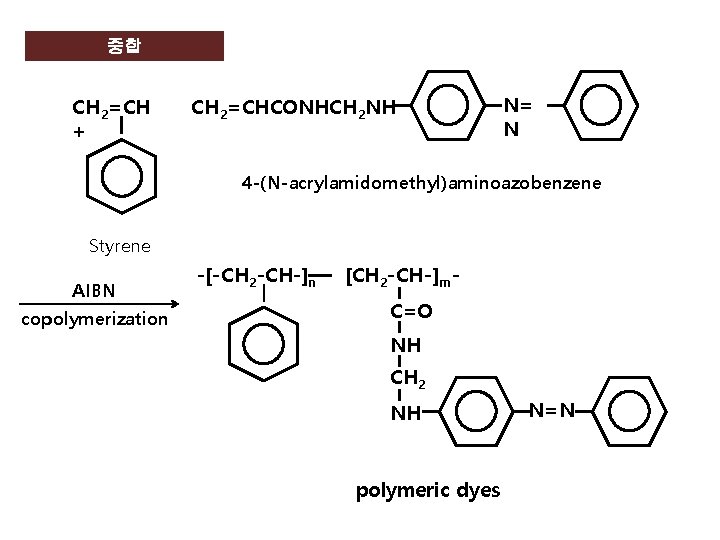
중합 CH 2=CH + CH 2=CHCONHCH 2 NH N= N 4 -(N-acrylamidomethyl)aminoazobenzene Styrene AIBN copolymerization -[-CH 2 -CH-]n [CH 2 -CH-]m. C=O NH CH 2 NH polymeric dyes N=N

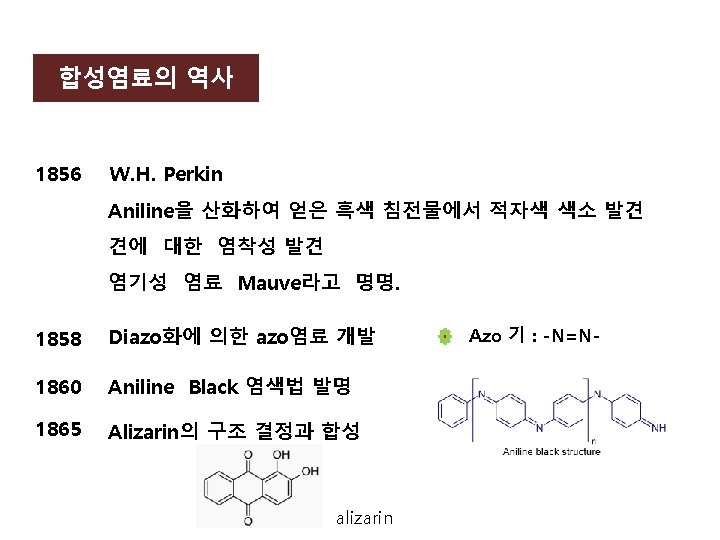
![Diazo화 과정 ArNH 2 Na NO 2HCl diazo화 ArN 2Cl ArNNAr azo염료 ArH coupling성분 Diazo화 과정 Ar-NH 2 Na. NO 2/HCl diazo화 Ar-N 2+]Cl- Ar-N=N-Ar’ azo염료 Ar’-H coupling성분](https://slidetodoc.com/presentation_image_h2/9b308ec72d912dfd1bc5e4cf20ebcfae/image-55.jpg)
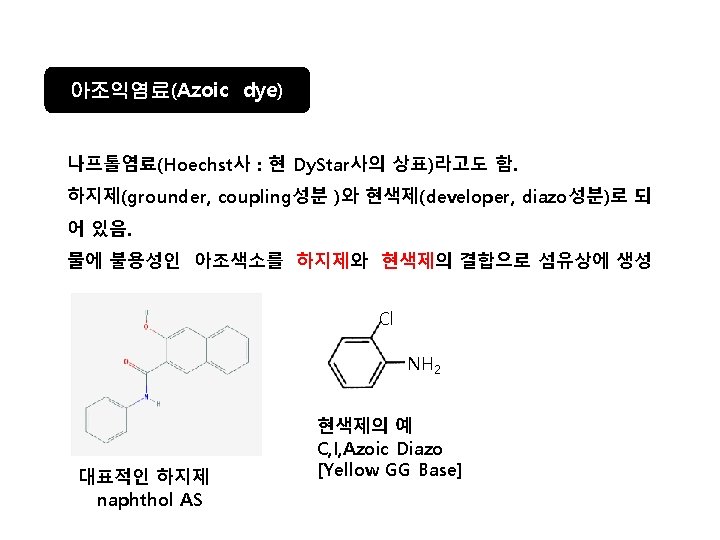
Diazo화 과정 Ar-NH 2 Na. NO 2/HCl diazo화 Ar-N 2+]Cl- Ar-N=N-Ar’ azo염료 Ar’-H coupling성분 HO Na. O 3 S SO 3 Na -N=NAmaranth(mono azo, soluble, acid dye) SO 3 Na

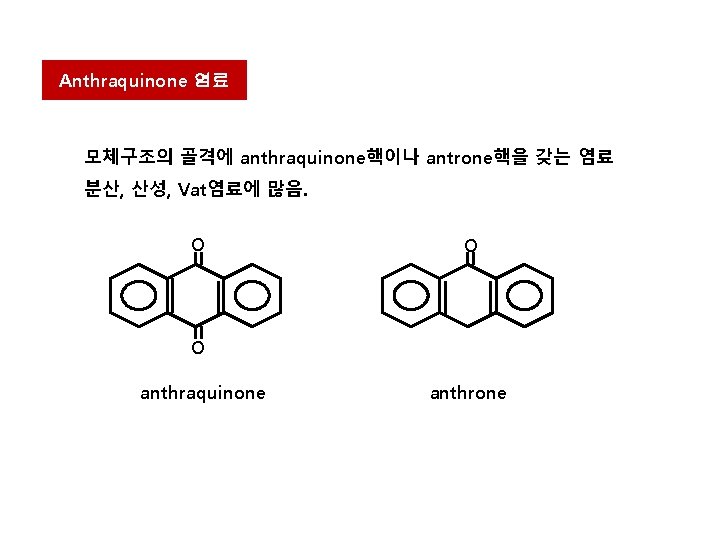
Anthraquinone 염료 모체구조의 골격에 anthraquinone핵이나 antrone핵을 갖는 염료 분산, 산성, Vat염료에 많음. O O O anthraquinone anthrone


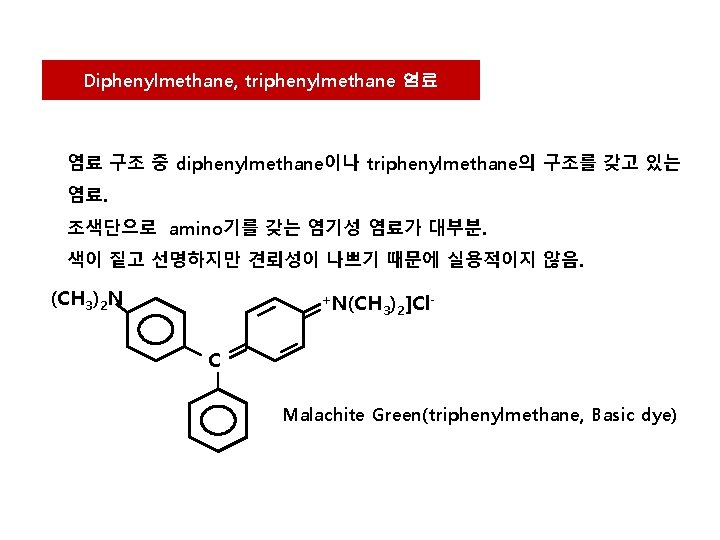
Diphenylmethane, triphenylmethane 염료 염료 구조 중 diphenylmethane이나 triphenylmethane의 구조를 갖고 있는 염료. 조색단으로 amino기를 갖는 염기성 염료가 대부분. 색이 짙고 선명하지만 견뢰성이 나쁘기 때문에 실용적이지 않음. (CH 3)2 N +N(CH 3)2]Cl - C Malachite Green(triphenylmethane, Basic dye)

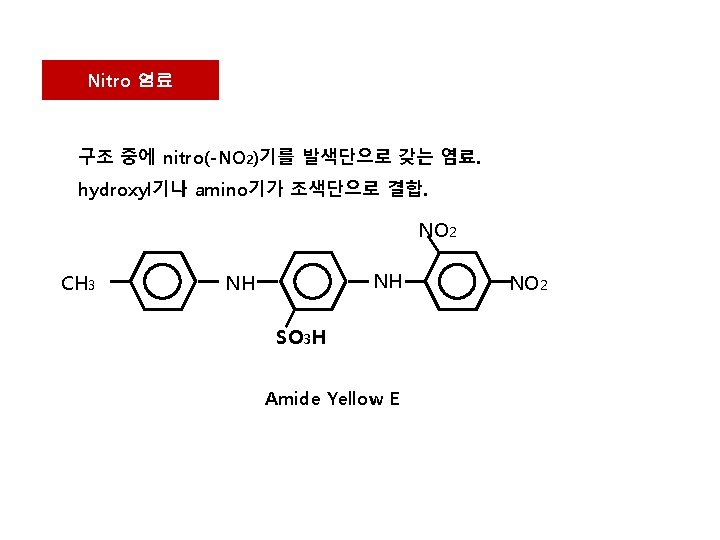
Nitro 염료 구조 중에 nitro(-NO 2)기를 발색단으로 갖는 염료. hydroxyl기나 amino기가 조색단으로 결합. NO 2 CH 3 NH NH SO 3 H Amide Yellow E NO 2

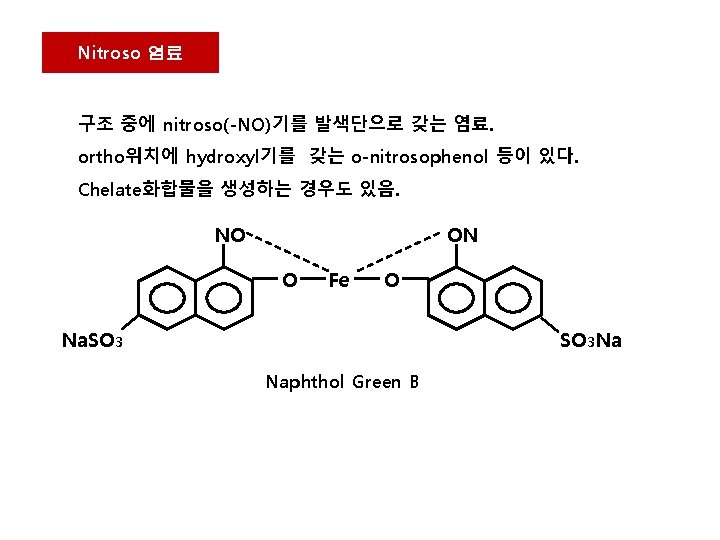
Nitroso 염료 구조 중에 nitroso(-NO)기를 발색단으로 갖는 염료. ortho위치에 hydroxyl기를 갖는 o-nitrosophenol 등이 있다. Chelate화합물을 생성하는 경우도 있음. NO ON O Fe O Na. SO 3 Na Naphthol Green B




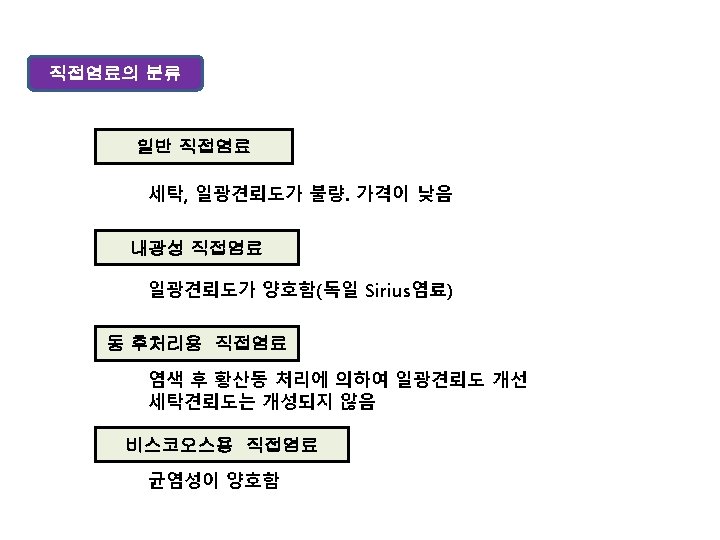
상품명에서의 중요한 관칭 예 염료 관칭 직접염료 Sirius, Sumlight, Kayarus, Solar, Chrysamine, Nippon 산성염료 Katanol, Polar, Solar, Diacid, Alizarine 염기성염료 Aizen, Diacryl, Para, Malachite, Janus 매염염료 Crome, Diamond 아조익염료 Bordeaux, Kako, Sanyo, Naphthol 황화염료 Kayasol, Sulphur, Carbanol Vat염료 Indigosol, Indanthrene, Vat 분산염료 Celliton, Dispersol, Foron 반응염료 Procion, Ciacron, Remazol, Sumifix




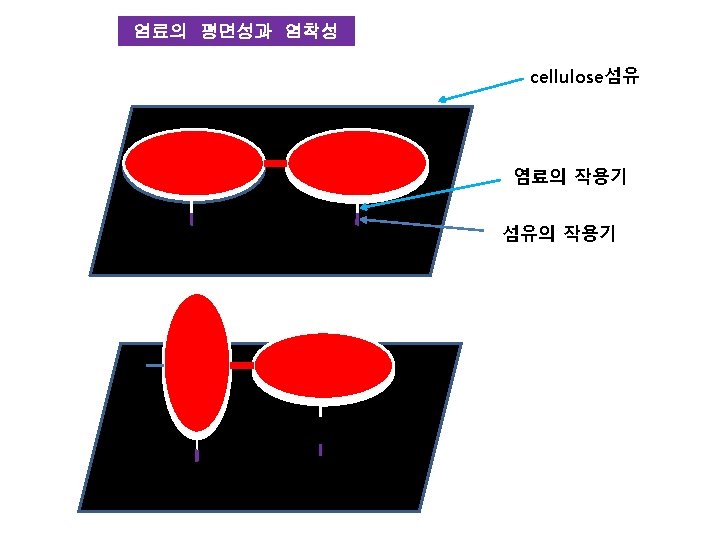
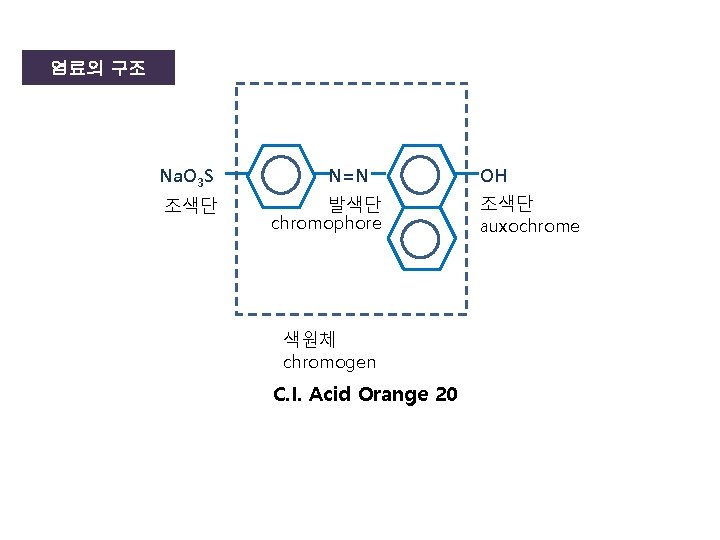
염료의 구조 Na. O 3 S 조색단 N=N 발색단 chromophore 색원체 chromogen C. I. Acid Orange 20 OH 조색단 auxochrome

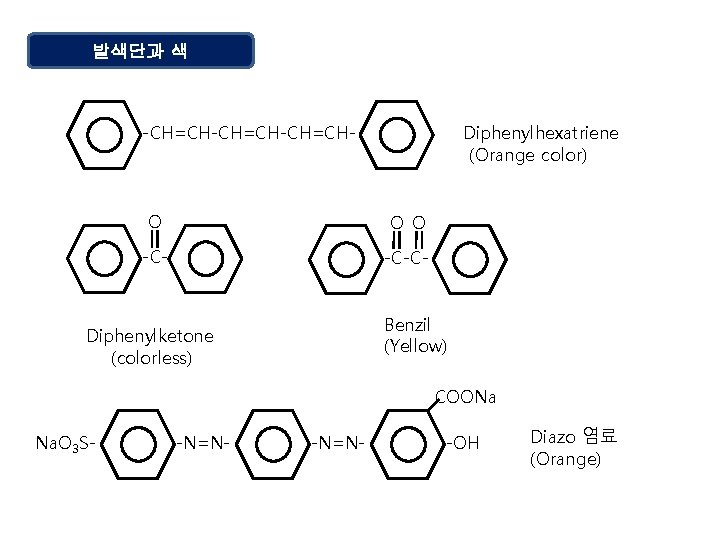
발색단과 색 -CH=CH-CH=CH- Diphenylhexatriene (Orange color) O O O -C-CBenzil (Yellow) Diphenylketone (colorless) COONa Na. O 3 S- -N=N- -OH Diazo 염료 (Orange)

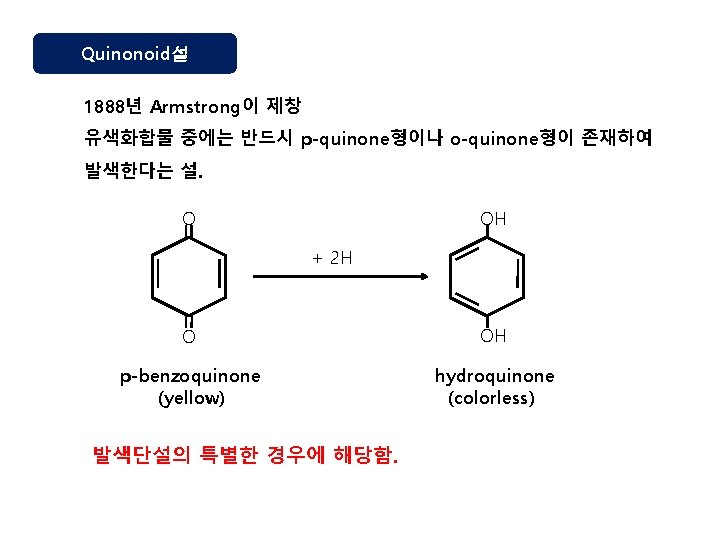
Quinonoid설 1888년 Armstrong이 제창 유색화합물 중에는 반드시 p-quinone형이나 o-quinone형이 존재하여 발색한다는 설. O OH + 2 H O OH p-benzoquinone (yellow) hydroquinone (colorless) 발색단설의 특별한 경우에 해당함.

