Theories of Acids and Bases Arrhenius Acid Substance

Theories of Acids and Bases

Arrhenius Acid • Substance that contains hydrogen & ionizes to produce H+ as the only positive ion in aqueous solution. • HCl(g) H+(aq) + Cl-(aq) • HNO 3 H+(aq) + NO 3 -(aq)
Arrhenius Base • A substance that contains a hydroxide group & ionizes to produce OH as the only negative ion in aqueous solution. • Na. OH(s) + Na (aq) + OH (aq)

Arrhenius Salt • Electrolytes where is not the only positive ion and OH is not the only negative ion in aq. solution. • Ex: Na. Cl, Ca. Br 2, KNO 3, NH 4 I + H

Salts in Water • Na. Cl(s) Na+(aq) + Cl-(aq) • Ca. Br 2(s) Ca+2(aq) + 2 Br-(aq) • KNO 3(s) K+(aq) + NO 3 -(aq) • NH 4 I(s) NH 4+(aq) + I-(aq)
Arrhenius Model has limitations • Don’t always use H 2 O as the solvent. • Arrhenius model only applies when H 2 O is the solvent. • Doesn’t explain all cases: – NH 3 doesn’t contain OH- but it produces OH-.

Alternate Theory: Bronsted-Lowry • Acid = a proton donor • All Arrhenius acids are Bronsted. Lowry Acids. • HX(g) + H 2 O(l) H 3 O+ + X • H+ forms a molecule-ion bond with the water molecule H 3 O+, named hydronium ion
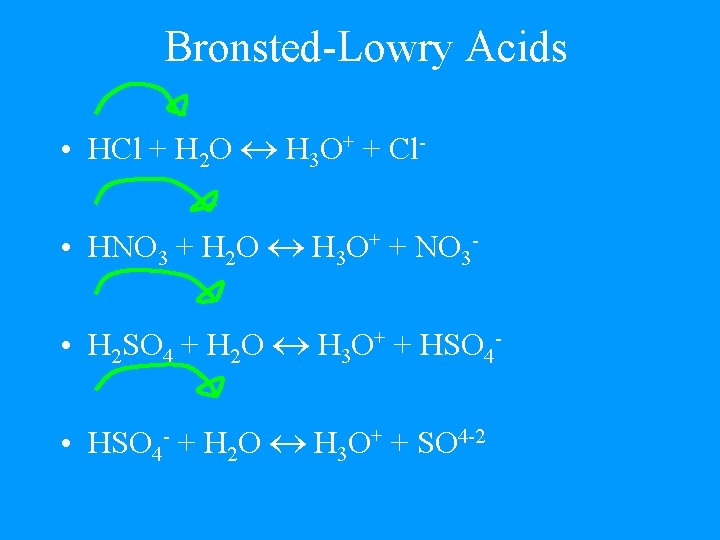
Bronsted-Lowry Acids • HCl + H 2 O H 3 O+ + Cl • HNO 3 + H 2 O H 3 O+ + NO 3 • H 2 SO 4 + H 2 O H 3 O+ + HSO 4 • HSO 4 - + H 2 O H 3 O+ + SO 4 -2
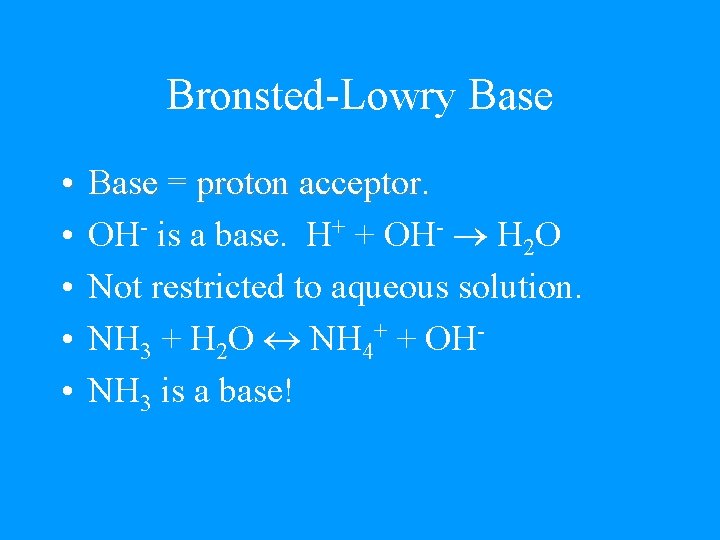
Bronsted-Lowry Base • • • Base = proton acceptor. OH- is a base. H+ + OH- H 2 O Not restricted to aqueous solution. NH 3 + H 2 O NH 4+ + OHNH 3 is a base!
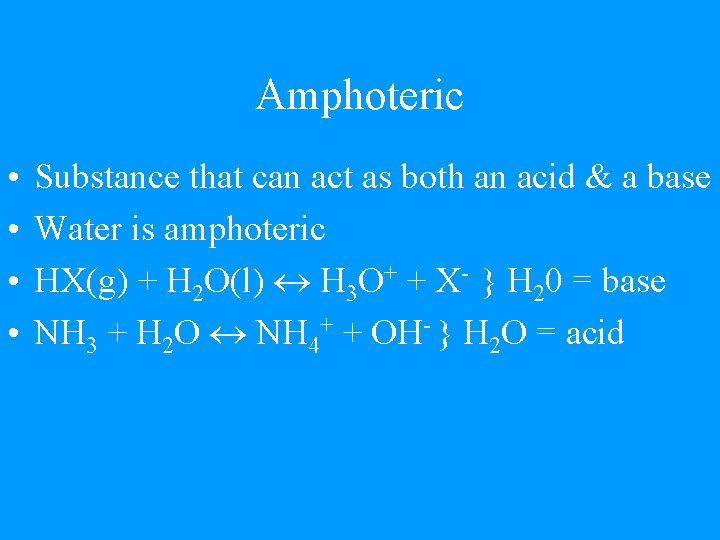
Amphoteric • • Substance that can act as both an acid & a base Water is amphoteric HX(g) + H 2 O(l) H 3 O+ + X- } H 20 = base NH 3 + H 2 O NH 4+ + OH- } H 2 O = acid
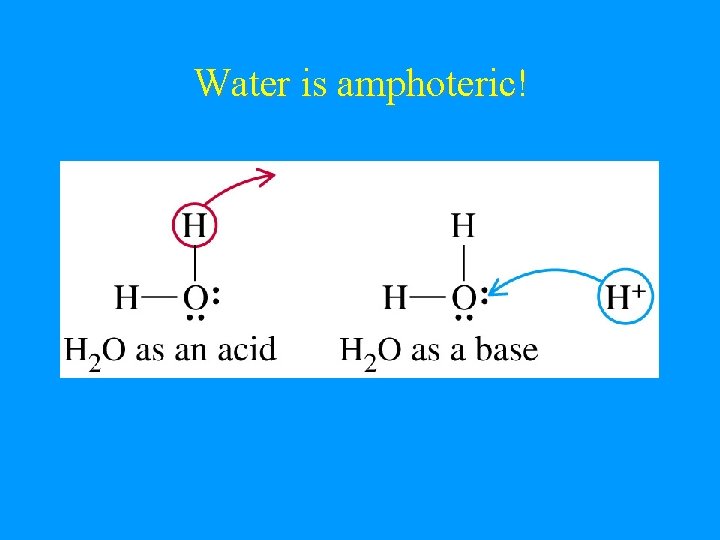
Water is amphoteric!

Conjugate Acid / Conjugate Base HX + H 2 O H 3 O+ + XAcid Base Conjugate Acid & Conjugate Base always on product side. Conjugate Acid = species that got the H+ Conjugate Base = species that lost the H+
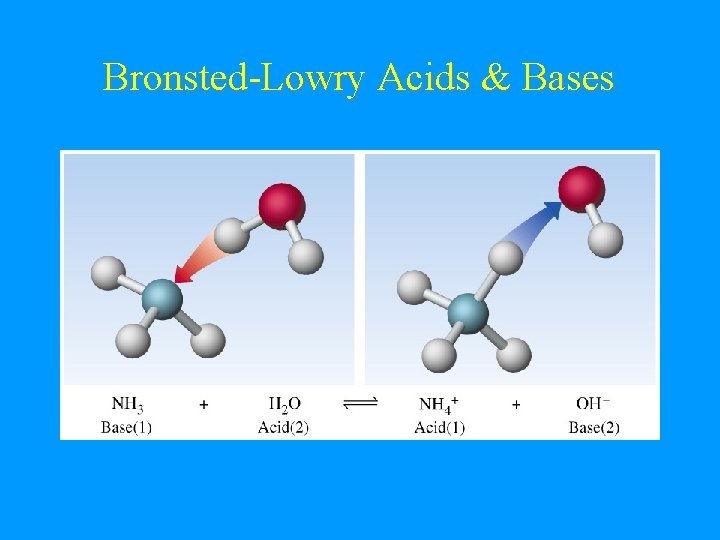
Bronsted-Lowry Acids & Bases
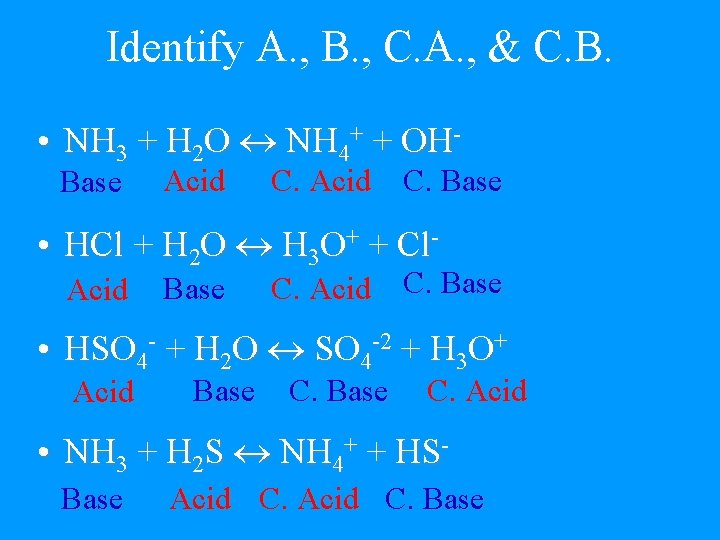
Identify A. , B. , C. A. , & C. B. • NH 3 + H 2 O NH 4+ + OHBase Acid C. Base • HCl + H 2 O H 3 O+ + Cl. Acid Base C. Acid C. Base • HSO 4 - + H 2 O SO 4 -2 + H 3 O+ Acid Base C. Acid • NH 3 + H 2 S NH 4+ + HSBase Acid C. Base

Lewis Definition • Lewis acid-base theory focuses on the exchange of electron pairs rather than protons to classify as acid or base. • Based on bonding and structure • Includes substances that do NOT contain hydrogen ions. • NH 3 + H 2 O NH 4+ + OH-

Lewis Acids & Bases • A Lewis acid is an atom, ion, or molecule that is an electron-pair acceptor. • Associate: – Bronsted-Lowry acid with: – Bronsted-Lowry base with: – Lewis acid with: – Lewis base with: proton donor proton acceptor electron pair donor
- Slides: 16