Theoretical Yield and Percent Yield HOW MUCH DID


- Slides: 10

Theoretical Yield and Percent Yield HOW MUCH DID YOU ACTUALLY GET?

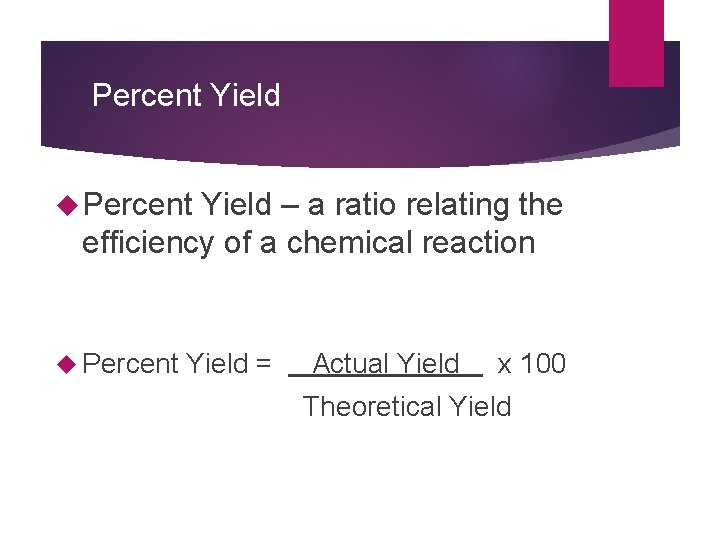
Percent Yield – a ratio relating the efficiency of a chemical reaction Percent Yield = Actual Yield x 100 Theoretical Yield

Percent Yield Actual Yield – What you actually got from the reaction/experiment Theoretical Yield – What you expected to get from the reaction/experiment Your ACTUAL YIELD should always be LESS than your THEORETICAL YIELD Nothing is PERFECT!

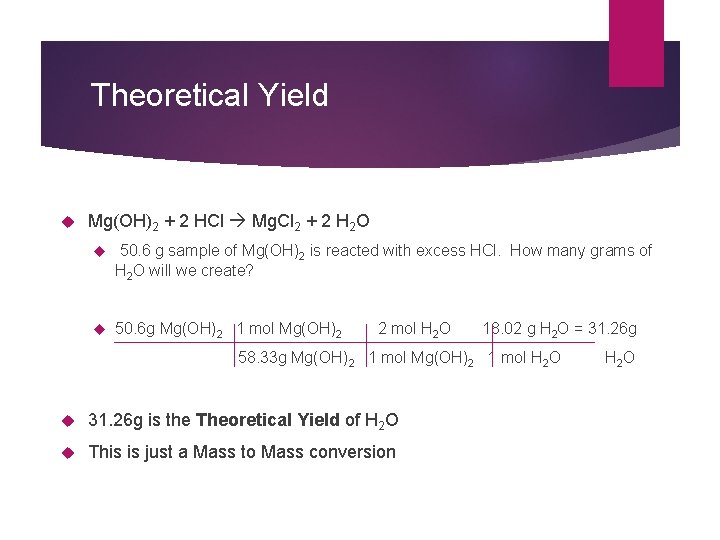
Theoretical Yield Mg(OH)2 + 2 HCl Mg. Cl 2 + 2 H 2 O 50. 6 g sample of Mg(OH)2 is reacted with excess HCl. How many grams of H 2 O will we create? 50. 6 g Mg(OH)2 1 mol Mg(OH)2 2 mol H 2 O 18. 02 g H 2 O = 31. 26 g 58. 33 g Mg(OH) 2 1 mol Mg(OH)2 1 mol H 2 O 31. 26 g is the Theoretical Yield of H 2 O This is just a Mass to Mass conversion

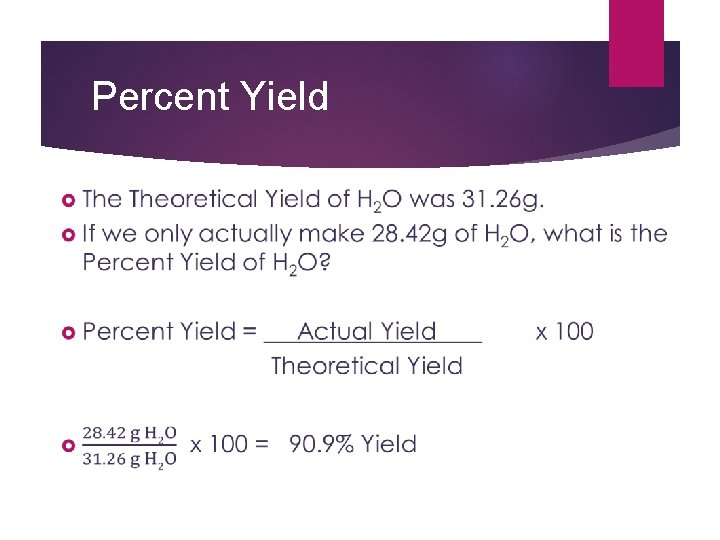
Percent Yield

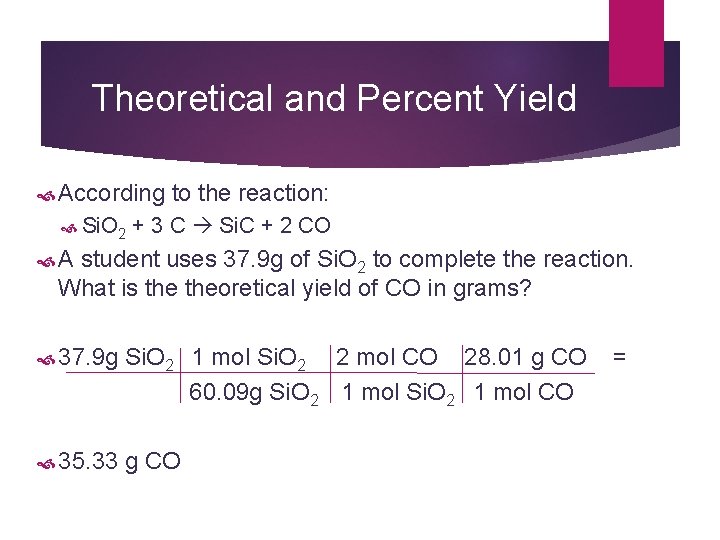
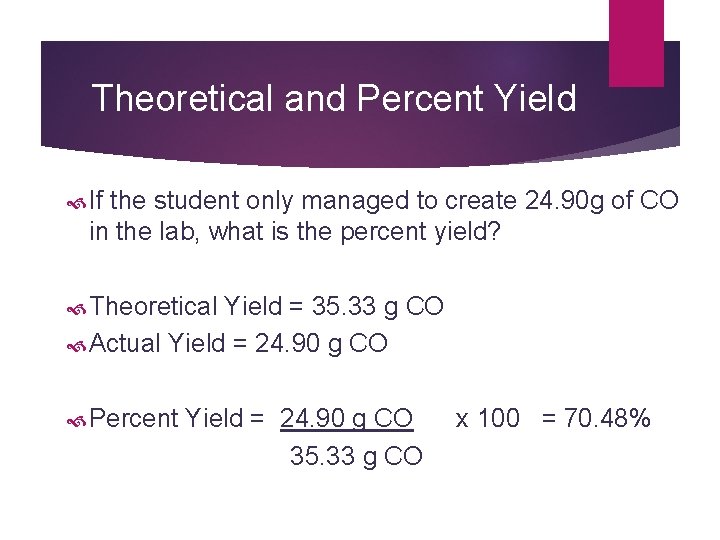
Theoretical and Percent Yield According to the reaction: Si. O 2 + 3 C Si. C + 2 CO A student uses 37. 9 g of Si. O 2 to complete the reaction. What is theoretical yield of CO in grams? 37. 9 g Si. O 2 1 mol Si. O 2 2 mol CO 28. 01 g CO = 60. 09 g Si. O 2 1 mol Si. O 2 1 mol CO 35. 33 g CO

Theoretical and Percent Yield If the student only managed to create 24. 90 g of CO in the lab, what is the percent yield? Theoretical Yield = 35. 33 g CO Actual Yield = 24. 90 g CO Percent Yield = 24. 90 g CO x 100 = 70. 48% 35. 33 g CO

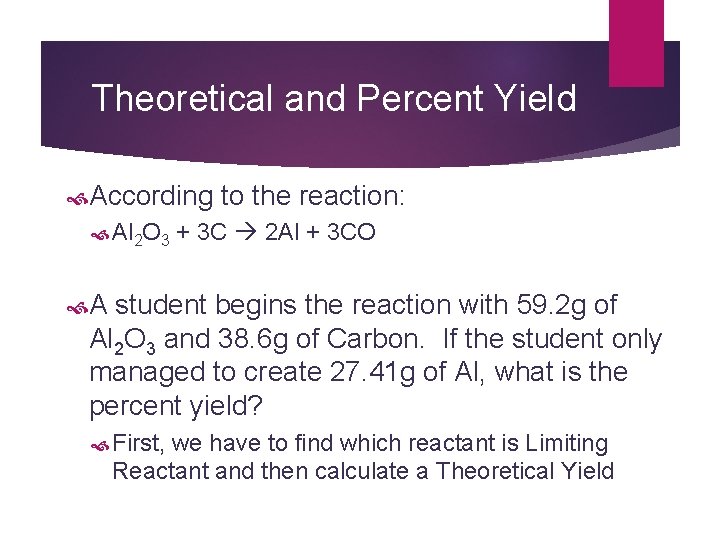
Theoretical and Percent Yield According to the reaction: Al 2 O 3 + 3 C 2 Al + 3 CO A student begins the reaction with 59. 2 g of Al 2 O 3 and 38. 6 g of Carbon. If the student only managed to create 27. 41 g of Al, what is the percent yield? First, we have to find which reactant is Limiting Reactant and then calculate a Theoretical Yield

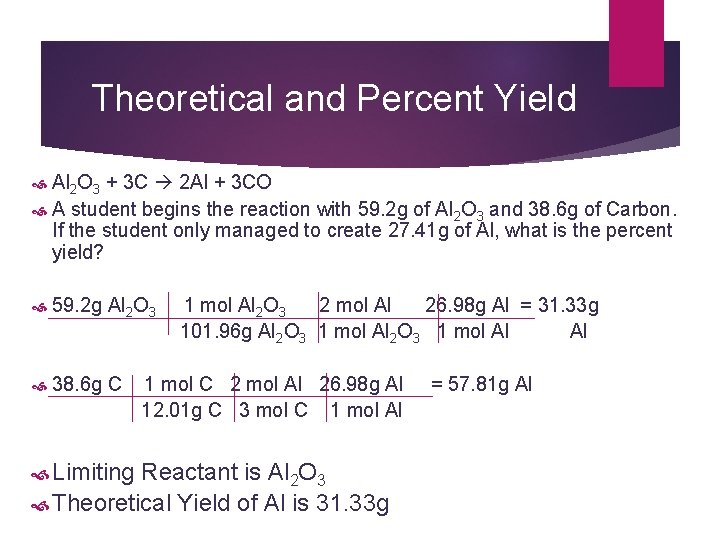
Theoretical and Percent Yield Al 2 O 3 + 3 C 2 Al + 3 CO A student begins the reaction with 59. 2 g of Al 2 O 3 and 38. 6 g of Carbon. If the student only managed to create 27. 41 g of Al, what is the percent yield? 59. 2 g Al 2 O 3 1 mol Al 2 O 3 2 mol Al 26. 98 g Al = 31. 33 g 101. 96 g Al 2 O 3 1 mol Al 2 O 3 1 mol Al 38. 6 g C 1 mol C 2 mol Al 26. 98 g Al = 57. 81 g Al 12. 01 g C 3 mol C 1 mol Al Limiting Reactant is Al 2 O 3 Theoretical Yield of Al is 31. 33 g

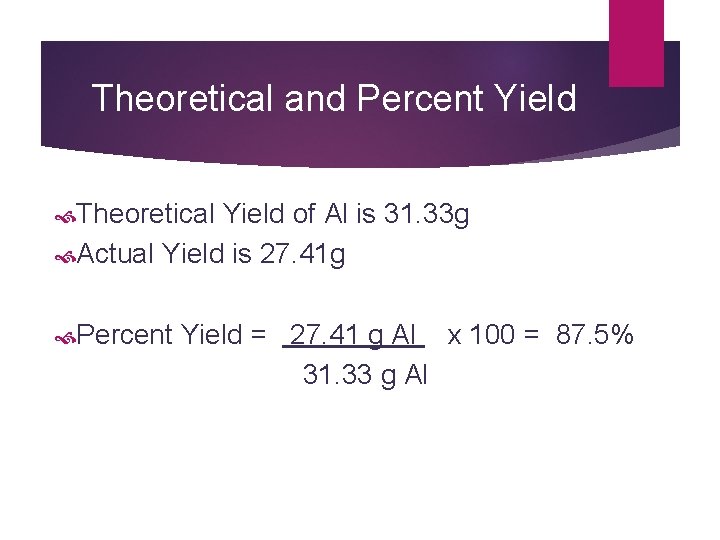
Theoretical and Percent Yield Theoretical Yield of Al is 31. 33 g Actual Yield is 27. 41 g Percent Yield = 27. 41 g Al x 100 = 87. 5% 31. 33 g Al