THEORETICAL STUDIES OF HIGHLY EXCITED VIBRATIONAL STATES USING

THEORETICAL STUDIES OF HIGHLY EXCITED VIBRATIONAL STATES USING VAN VLECK PERTURBATION THEORY University of Wisconsin Ned Sibert, Anne Mc. Coy, Darin Burleigh, Sai Ramesh, Xiaogang Wang, Marc Joyeaux, Christoph Iung, and Christof Jung

On σ-Type Doubling and Electron Spin in the Spectra of Diatomic Molecules J. H. Van Vleck Department of Physics, University of Wisconsin Received 31 January 1929
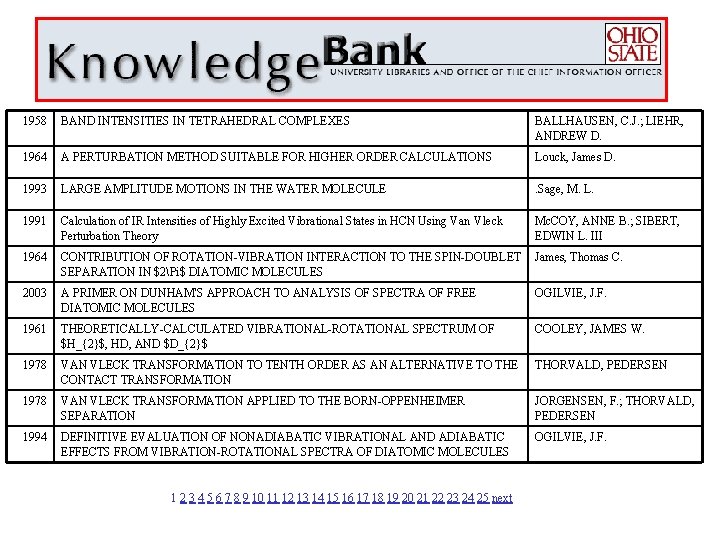
1958 BAND INTENSITIES IN TETRAHEDRAL COMPLEXES BALLHAUSEN, C. J. ; LIEHR, ANDREW D. 1964 A PERTURBATION METHOD SUITABLE FOR HIGHER ORDER CALCULATIONS Louck, James D. 1993 LARGE AMPLITUDE MOTIONS IN THE WATER MOLECULE . Sage, M. L. 1991 Calculation of IR Intensities of Highly Excited Vibrational States in HCN Using Van Vleck Perturbation Theory Mc. COY, ANNE B. ; SIBERT, EDWIN L. III 1964 CONTRIBUTION OF ROTATION-VIBRATION INTERACTION TO THE SPIN-DOUBLET SEPARATION IN $2Pi$ DIATOMIC MOLECULES James, Thomas C. 2003 A PRIMER ON DUNHAM'S APPROACH TO ANALYSIS OF SPECTRA OF FREE DIATOMIC MOLECULES OGILVIE, J. F. 1961 THEORETICALLY-CALCULATED VIBRATIONAL-ROTATIONAL SPECTRUM OF $H_{2}$, HD, AND $D_{2}$ COOLEY, JAMES W. 1978 VAN VLECK TRANSFORMATION TO TENTH ORDER AS AN ALTERNATIVE TO THE CONTACT TRANSFORMATION THORVALD, PEDERSEN 1978 VAN VLECK TRANSFORMATION APPLIED TO THE BORN-OPPENHEIMER SEPARATION JORGENSEN, F. ; THORVALD, PEDERSEN 1994 DEFINITIVE EVALUATION OF NONADIABATIC VIBRATIONAL AND ADIABATIC EFFECTS FROM VIBRATION-ROTATIONAL SPECTRA OF DIATOMIC MOLECULES OGILVIE, J. F. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 next
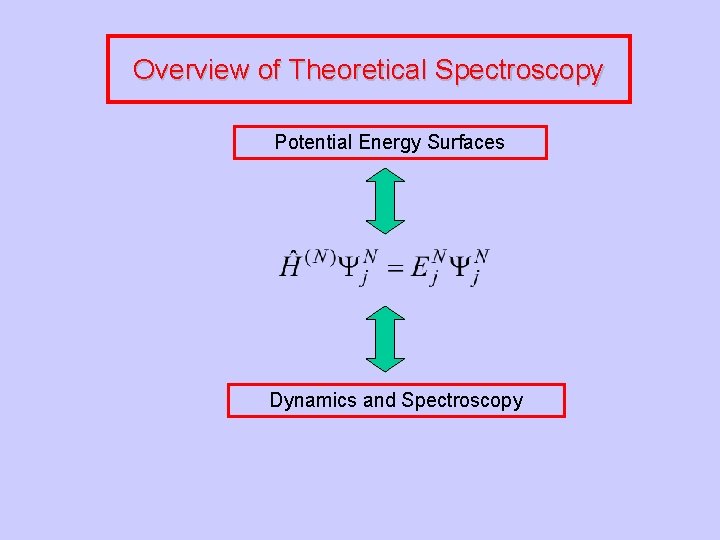
Overview of Theoretical Spectroscopy Potential Energy Surfaces Dynamics and Spectroscopy
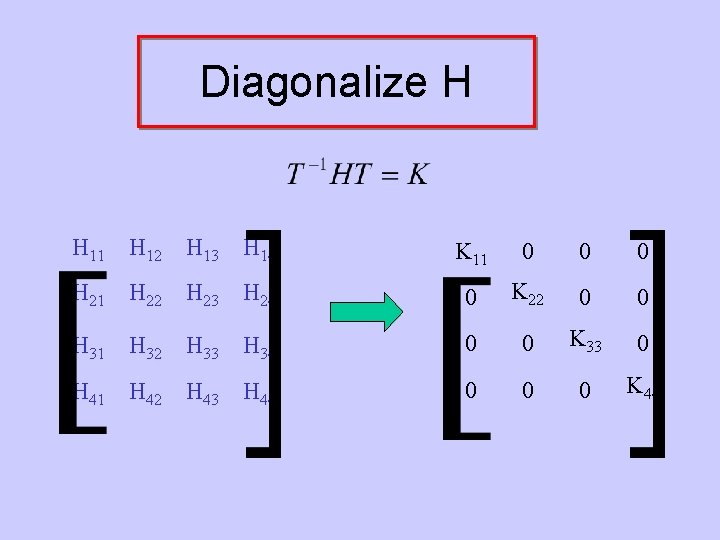
Diagonalize H H 11 H 12 H 13 H 14 K 11 0 0 0 H 21 H 22 H 23 H 24 0 K 22 0 0 H 31 H 32 H 33 H 34 0 0 K 33 0 H 41 H 42 H 43 H 44 0 0 0 K 44
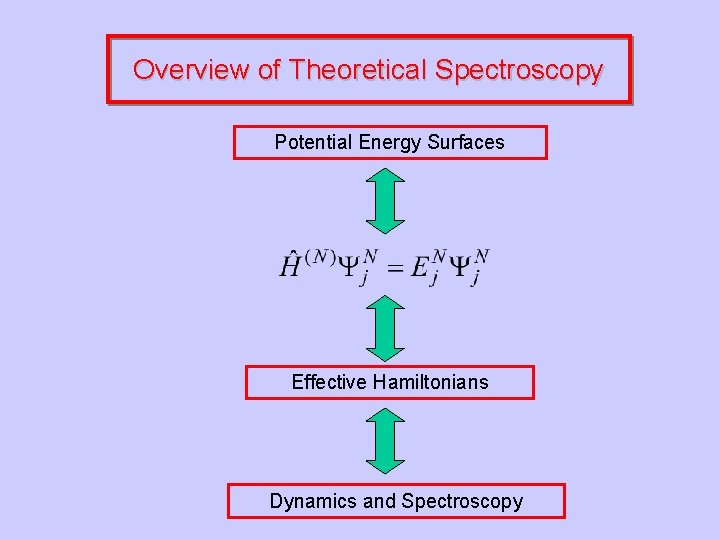
Overview of Theoretical Spectroscopy Potential Energy Surfaces Effective Hamiltonians Dynamics and Spectroscopy

FE 08 Effective Vibrational Rotational Hamiltonian in the Presence of a Stark Field B. Ram Prasad and Mangala Sunder Krishnan The standard theory for analysing high resolution Vibrational-Rotational. Torsional spectra of semi-rigid and non-rigid molecules is based on perturbation theory, which leads to the concept of effective Hamiltonians. Though there are several ways of obtaining molecular Hamiltonians, the method proposed by Van Vleck has proven to be quite useful in the area of molecular spectroscopy.
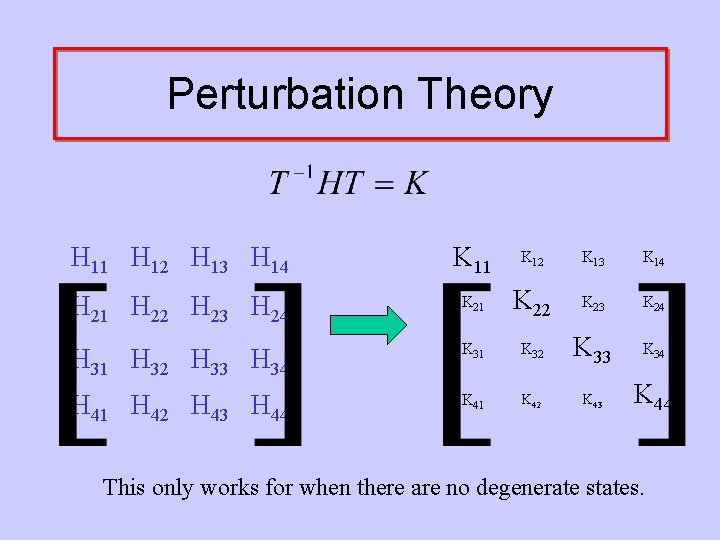
Perturbation Theory H 11 H 12 H 13 H 14 K 11 K 12 K 13 K 14 H 21 H 22 H 23 H 24 K 21 K 22 K 23 K 24 H 31 H 32 H 33 H 34 K 31 K 32 K 33 K 34 H 41 H 42 H 43 H 44 K 41 K 42 K 43 K 44 This only works for when there are no degenerate states.
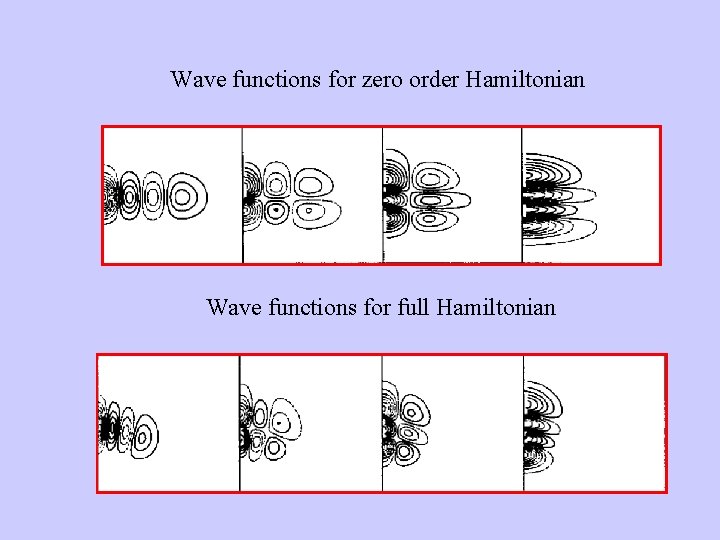
Wave functions for zero order Hamiltonian Wave functions for full Hamiltonian
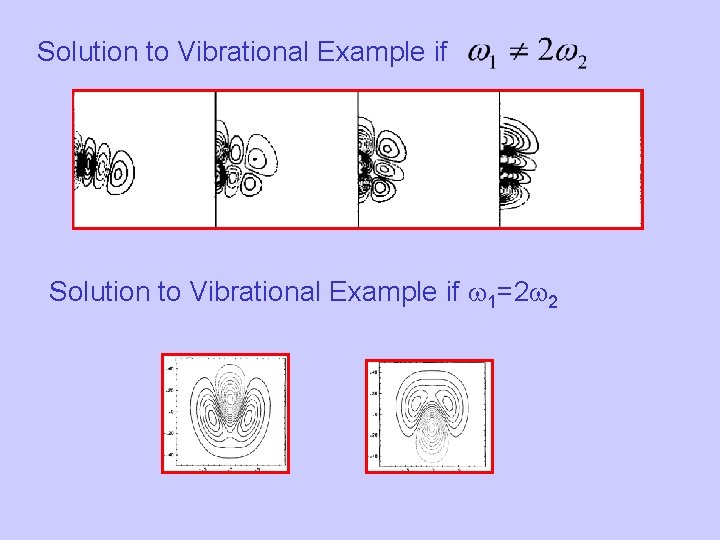
Solution to Vibrational Example if w 1=2 w 2
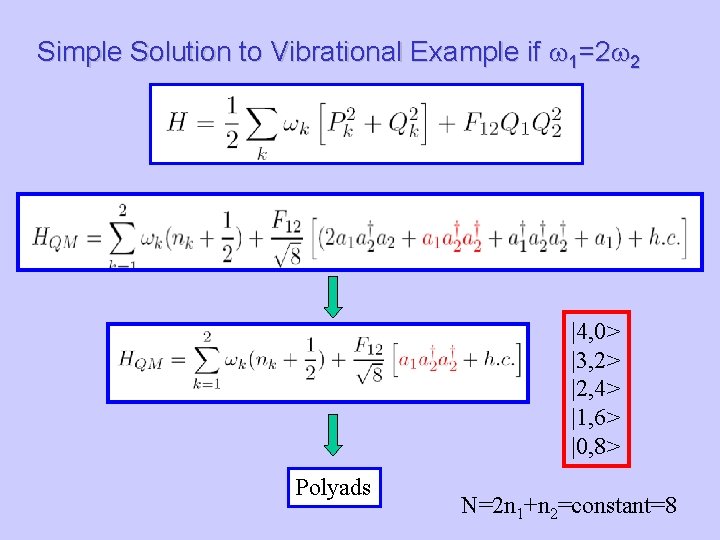
Simple Solution to Vibrational Example if w 1=2 w 2 |4, 0> |3, 2> |2, 4> |1, 6> |0, 8> Polyads N=2 n 1+n 2=constant=8
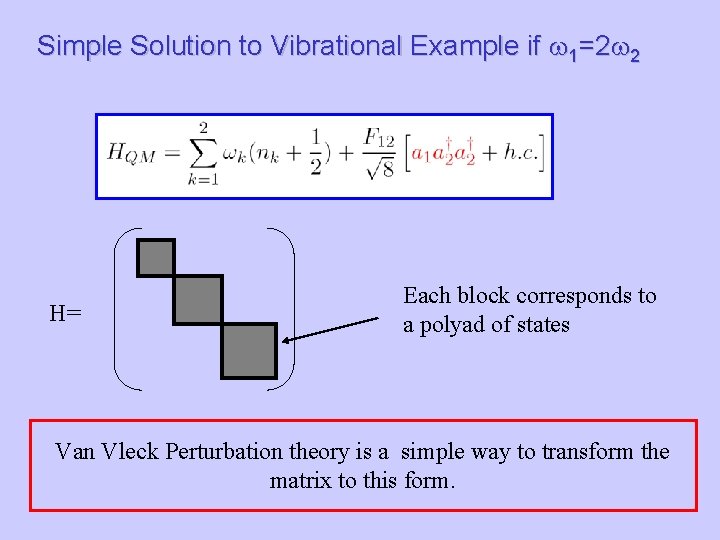
Simple Solution to Vibrational Example if w 1=2 w 2 H= Each block corresponds to a polyad of states Van Vleck Perturbation theory is a simple way to transform the matrix to this form.
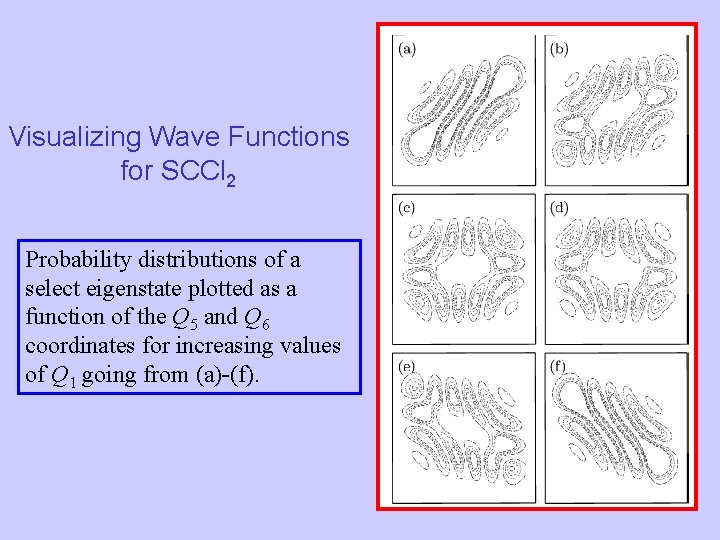
Visualizing Wave Functions for SCCl 2 Probability distributions of a select eigenstate plotted as a function of the Q 5 and Q 6 coordinates for increasing values of Q 1 going from (a)-(f).
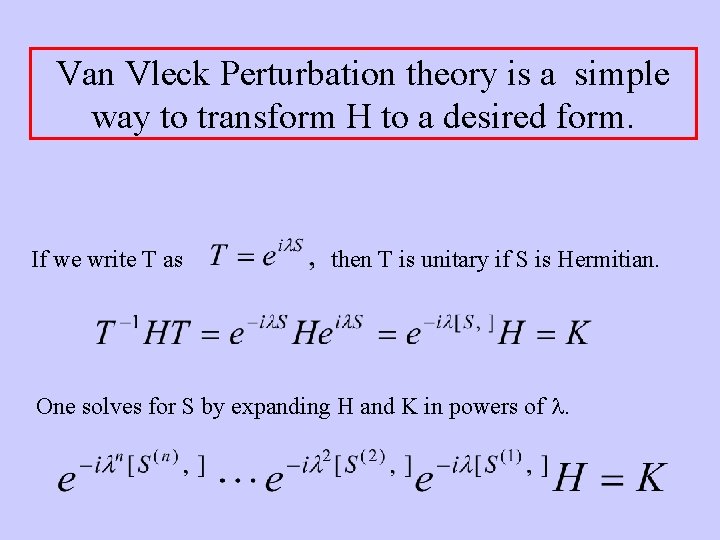
Van Vleck Perturbation theory is a simple way to transform H to a desired form. If we write T as then T is unitary if S is Hermitian. One solves for S by expanding H and K in powers of l.
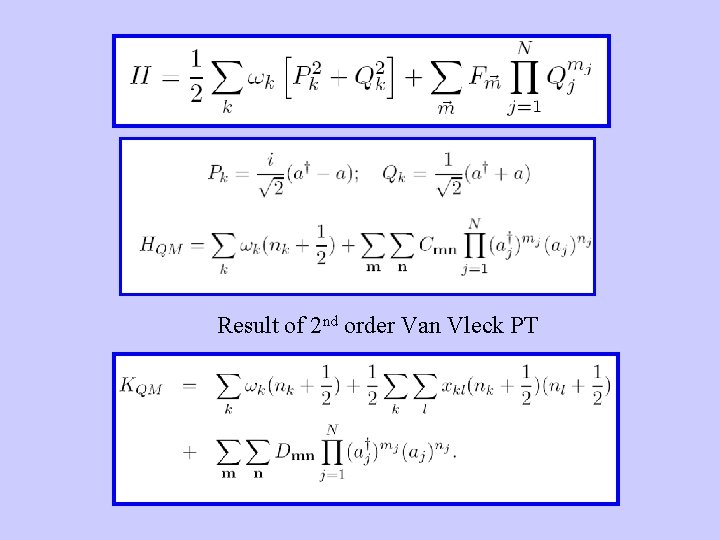
Result of 2 nd order Van Vleck PT
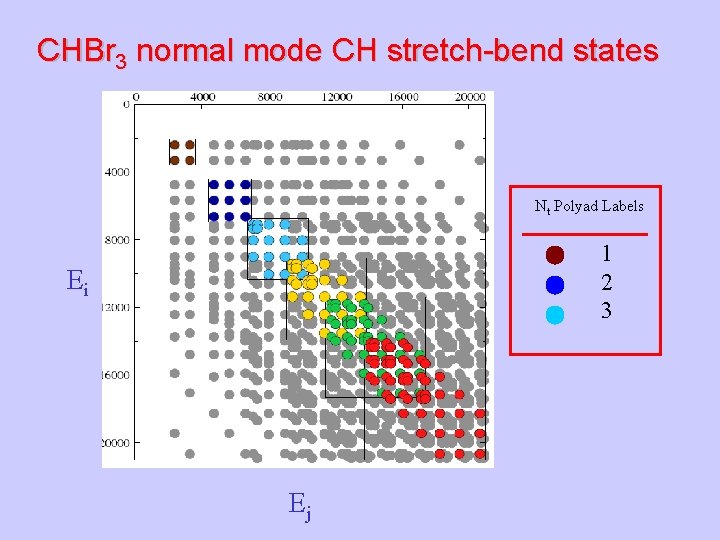
CHBr 3 normal mode CH stretch-bend states Nt Polyad Labels 1 2 3 Ei Ej
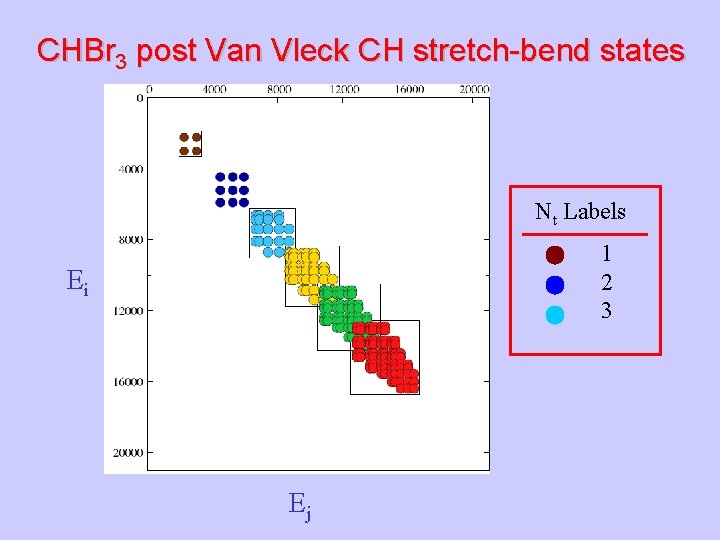
CHBr 3 post Van Vleck CH stretch-bend states Nt Labels 1 2 3 Ei Ej
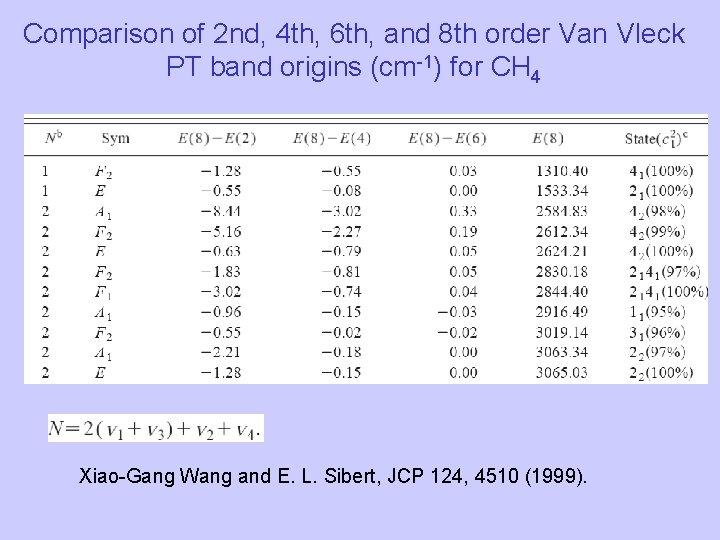
Comparison of 2 nd, 4 th, 6 th, and 8 th order Van Vleck PT band origins (cm-1) for CH 4 Xiao-Gang Wang and E. L. Sibert, JCP 124, 4510 (1999).

Comparison of 2 nd, 4 th, 6 th, and 8 th order Van Vleck PT band origins (cm-1) for CH 4 We are pushing the limits of CVPT so that it can be used to accurately calculate highly excited states.
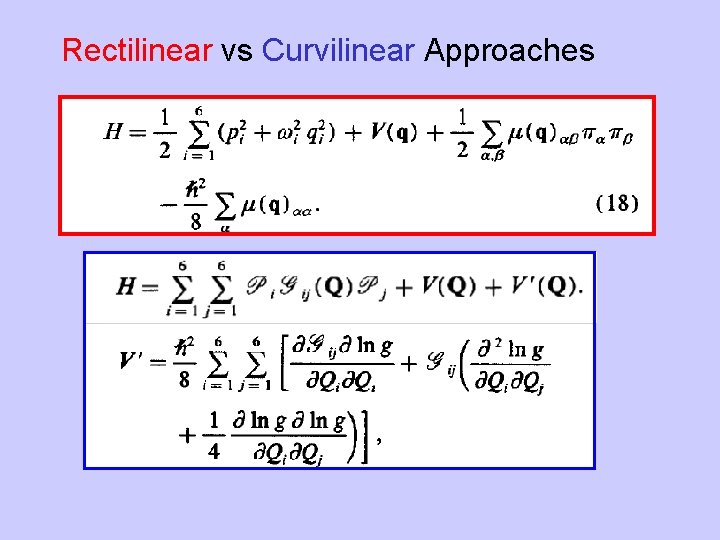
Rectilinear vs Curvilinear Approaches
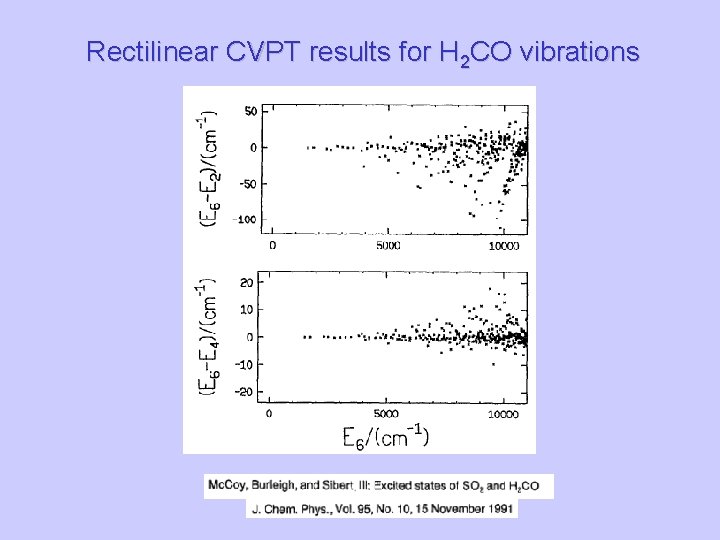
Rectilinear CVPT results for H 2 CO vibrations
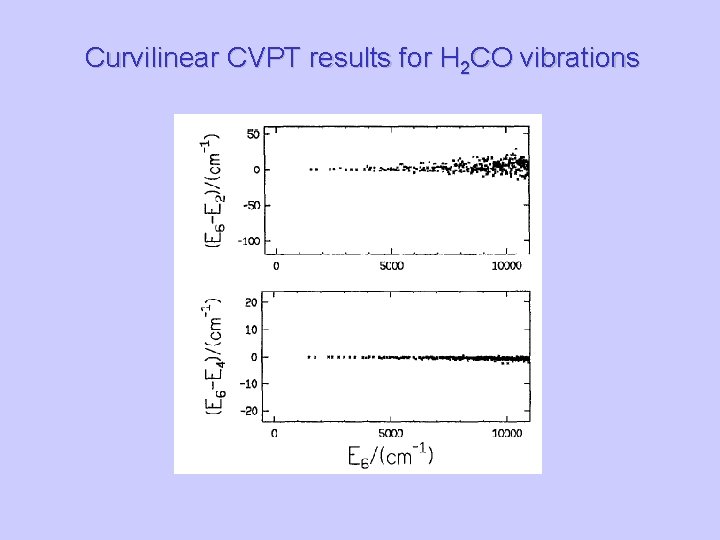
Curvilinear CVPT results for H 2 CO vibrations

Two Current Research Directions 1) Vibrations coupled to additional DOF 2) Creating good basis sets
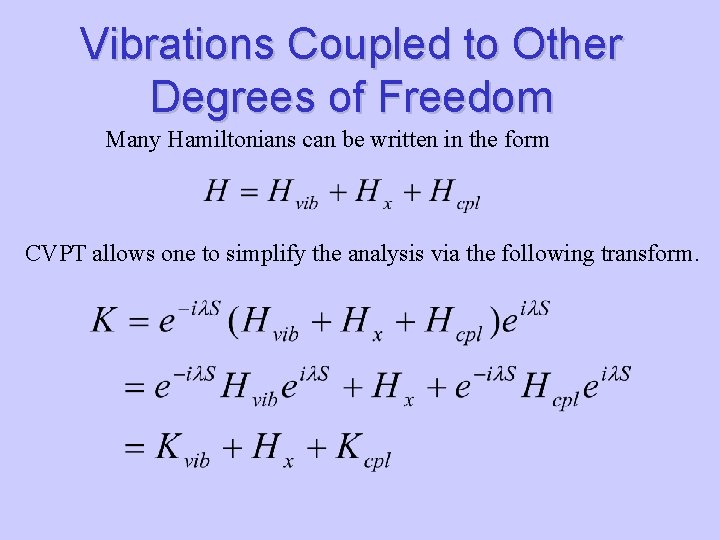
Vibrations Coupled to Other Degrees of Freedom Many Hamiltonians can be written in the form CVPT allows one to simplify the analysis via the following transform.

Vibrations coupled to other DOF Hamiltonians that can be written in the form include: ØRotation-vibration of H 2 O, H 2 CO, SO 2, CH 4, Ølaser-molecule for HCN, H 2 O, H 2 CO, CHBr 3, CHF 3 ØIsomerization HCN Øvibration-torsion CH 3 OH and deuterated analogues Øcondensed phase vibrational relaxation
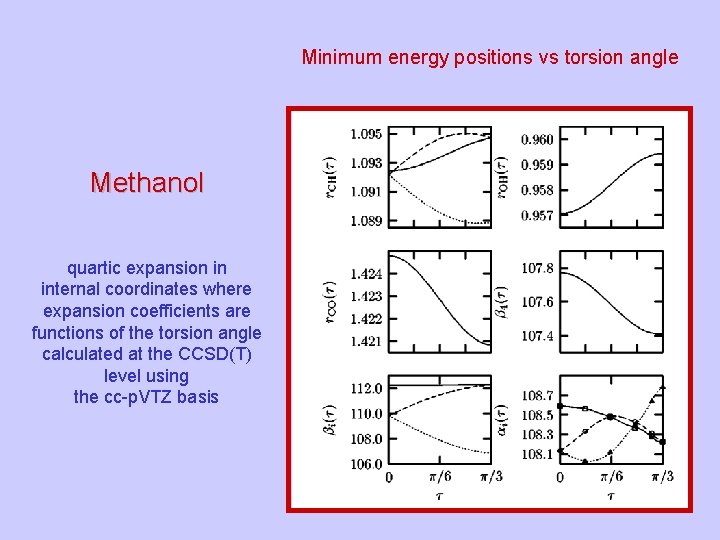
Minimum energy positions vs torsion angle Methanol quartic expansion in internal coordinates where expansion coefficients are functions of the torsion angle calculated at the CCSD(T) level using the cc-p. VTZ basis

Basic Approach
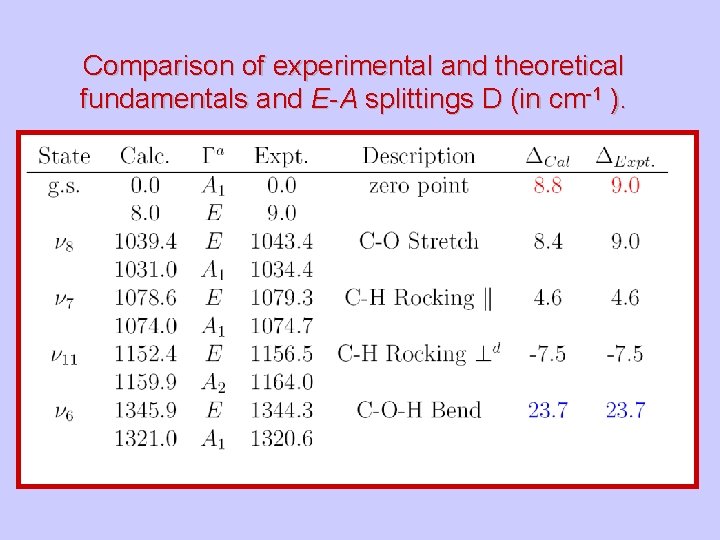
Comparison of experimental and theoretical fundamentals and E-A splittings D (in cm-1 ).
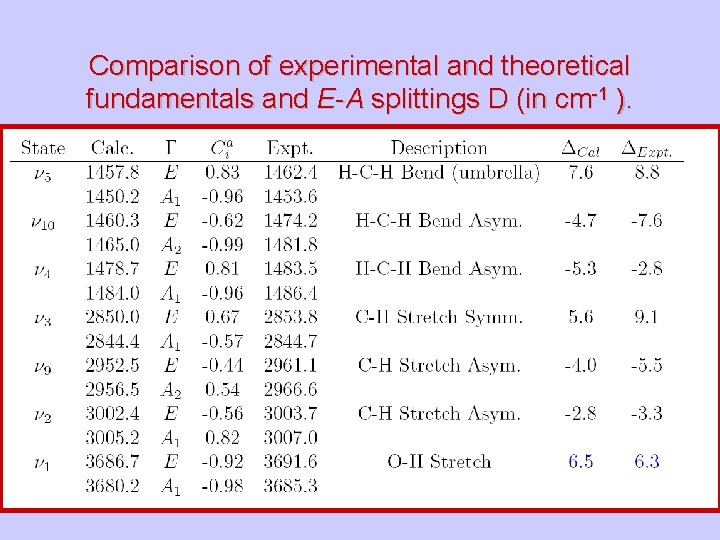
Comparison of experimental and theoretical fundamentals and E-A splittings D (in cm-1 ).

Creating Good Basis Sets Molecular Physics, Vol. 103, (2005), 149– 162. SAI G. RAMESH and EDWIN L. SIBERT
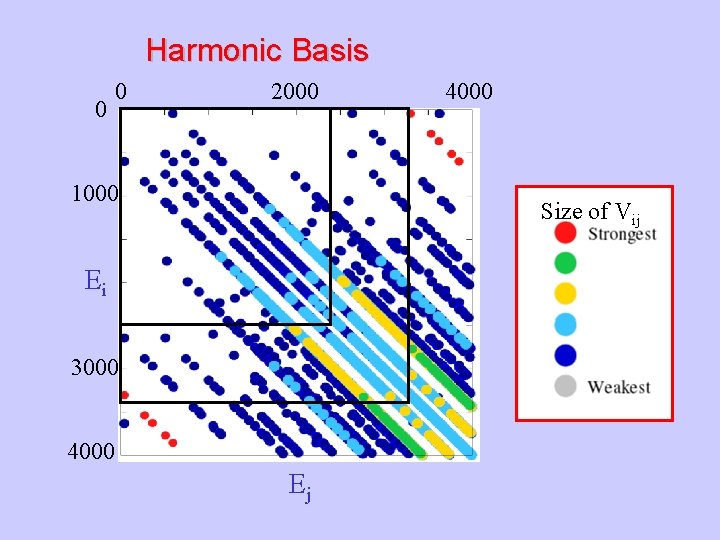
Harmonic Basis 0 0 2000 1000 4000 Size of Vij Ei 3000 4000 Ej
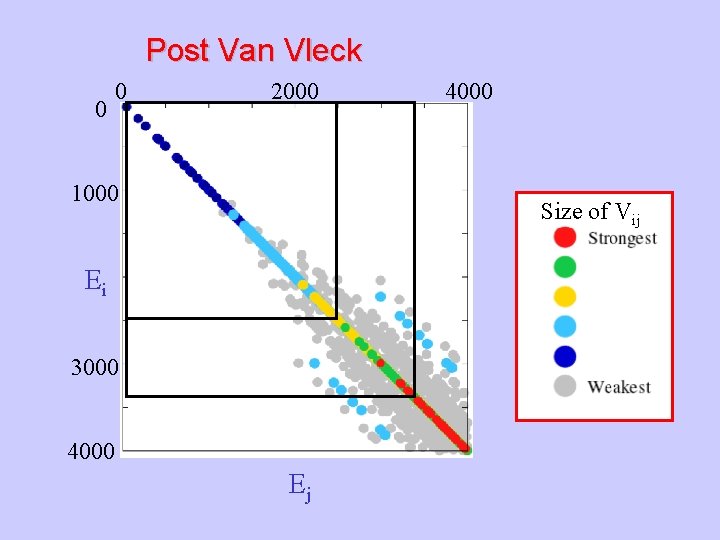
Post Van Vleck 0 0 2000 1000 4000 Size of Vij Ei 3000 4000 Ej
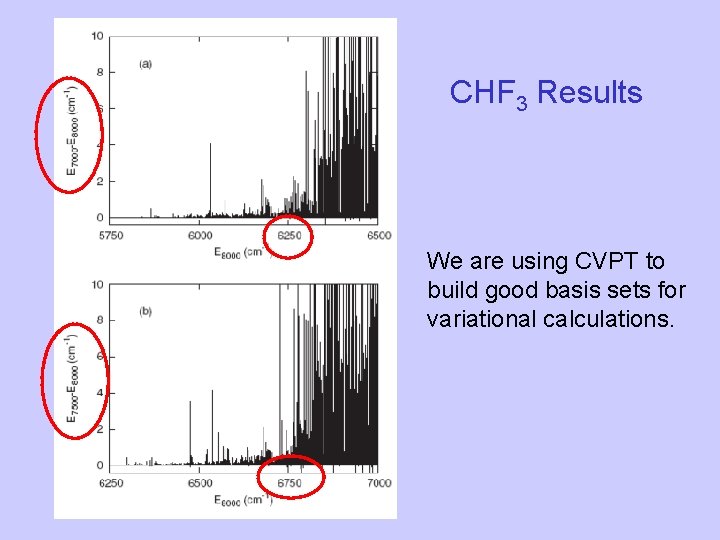
CHF 3 Results We are using CVPT to build good basis sets for variational calculations.
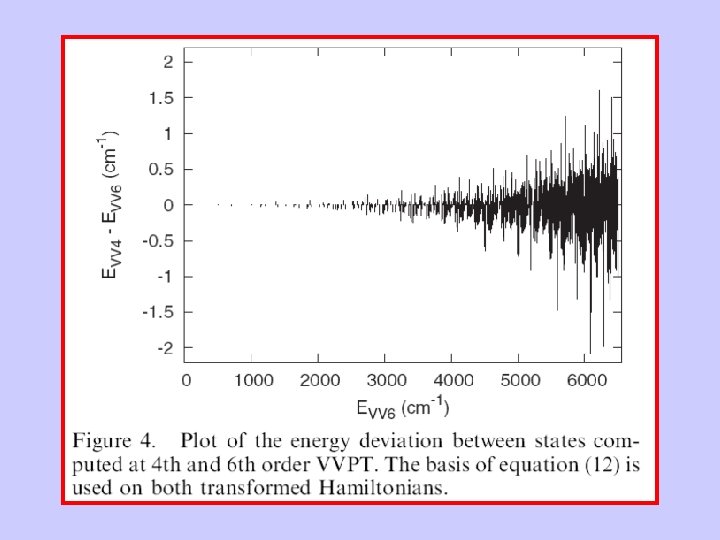
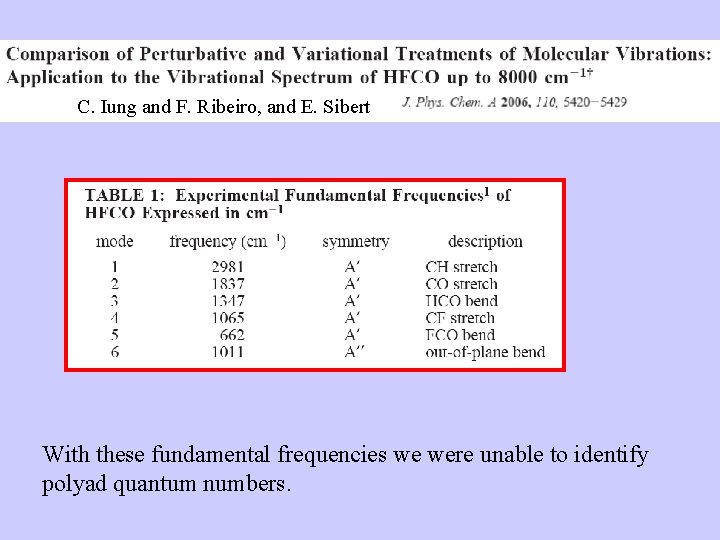
C. Iung and F. Ribeiro, and E. Sibert With these fundamental frequencies we were unable to identify polyad quantum numbers.
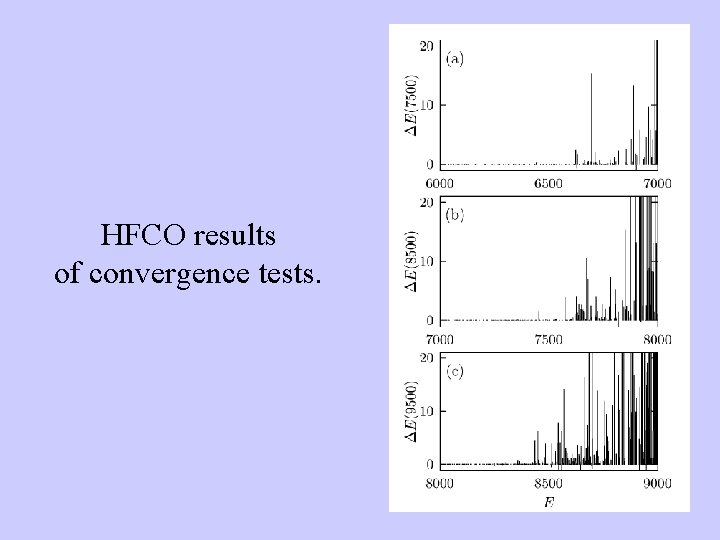
HFCO results of convergence tests.
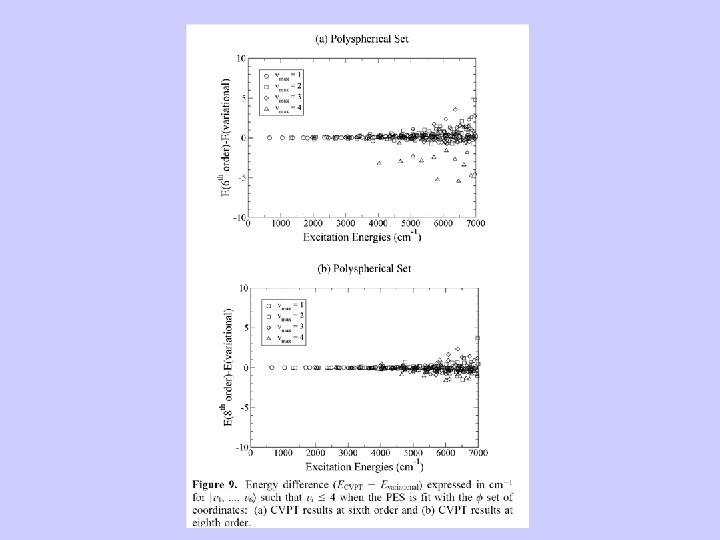
Conclusions Van Vleck perturbation theory remains an important tool for treating molecular vibrations as well as vibrations coupled to many other DOF. A study of the vibrations of fluoroform with a sixth order nine-dimensional potential: a combined perturbative-variational approach SAI G. RAMESH and E. L. SIBERT, Mol. Phys, 103, 2005, 149– 162 Quantum, semiclassical and classical dynamics of the bending modes of acetylene E. L. Sibert and Anne B. Mc. Coy JCP (1996)

The Sibert Group
- Slides: 41