Theoretical Inorganic Chemistry Group Structure and dynamics of










- Slides: 40

Theoretical Inorganic Chemistry Group Structure and dynamics of -cyclodextrin and glycine at quantum mechanical level Hélio A. Duarte, Hélio F. Dos Santos, Thomas Heine, Serguei Patchkovskii duarteh@ufmg. br Department of Chemistry - ICEx, Federal University of Minas Gerais - UFMG ACS 232 nd National Meeting San Francisco, CA - USA 1

Outline • Motivation • Spironolactone and its Complexes with -cyclodextrin • -cyclodextrine in aqueous solution – molecular dynamics using DFTB/MM approach. • Glycine in aqueous solution – molecular dynamics using full DFTB. 2

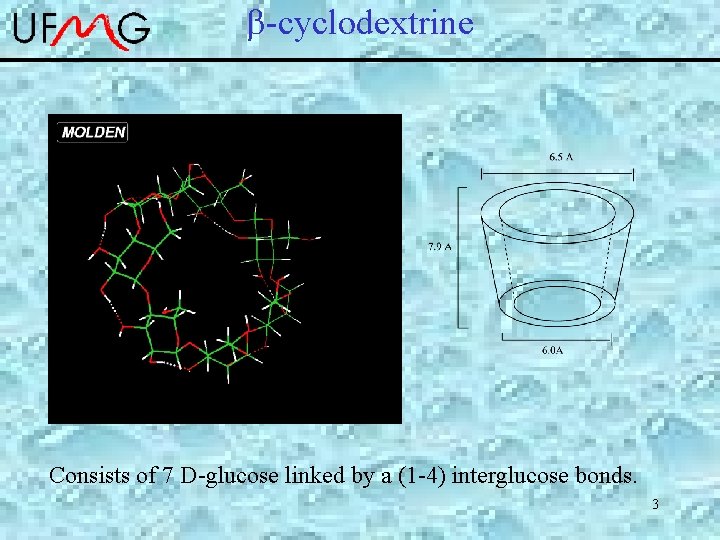
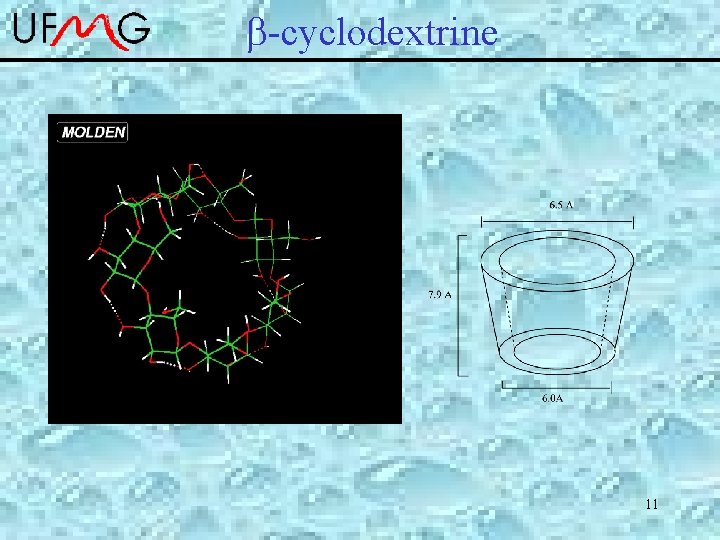
-cyclodextrine Consists of 7 D-glucose linked by a (1 -4) interglucose bonds. 3

b-Cyclodextrine • • Inclusion compounds Drug Delivery Systems Improved molecular switches Artificial enzymes Rotaxamers Nanoreactors Self-assembling systems 4

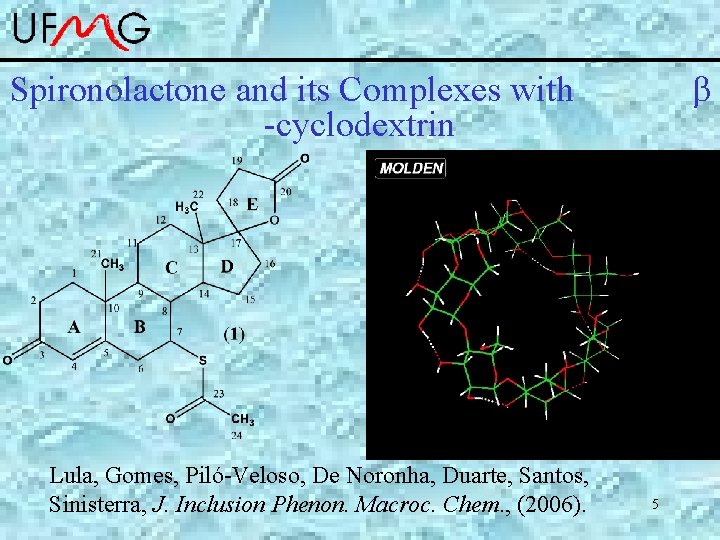
Spironolactone and its Complexes with -cyclodextrin Lula, Gomes, Piló-Veloso, De Noronha, Duarte, Santos, Sinisterra, J. Inclusion Phenon. Macroc. Chem. , (2006). 5

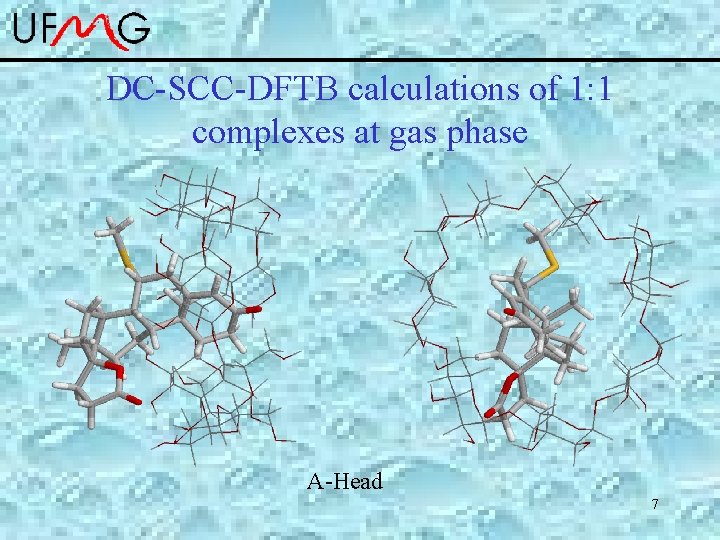
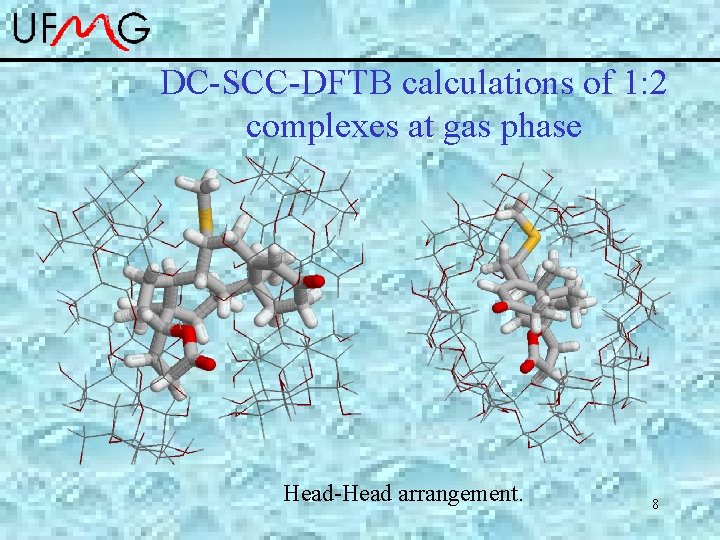
Spironolactone : -cyclodextrin • Complexes 1: 1 and 1: 2 are formed and well characterized by ROESY-NMR. • The rings A and DE are involved in the inclusion process. Simulation at gas phase: DC-SCC-DFTB *Zhechkov, L. ; Heine, T. ; Patchkovskii, S. ; Seifert, G. ; Duarte, H. A. JCTC 2005, 1, 841. *Elstner, et al. , Phys. Rev. B, 1998, 58, 7260. *Porezag, D. ; Frauenheim, T. ; Kohler, T. ; Seifert, G. ; Kaschner, R. Physical 6 Review B 1995, 51, 12947

DC-SCC-DFTB calculations of 1: 1 complexes at gas phase A-Head 7

DC-SCC-DFTB calculations of 1: 2 complexes at gas phase Head-Head arrangement. 8

Inclusion process: guest: host according to Rekharsky and Inoue, Chem. Rev. , 98, 1875. 1. penetration of the hydrophobic part of the guest molecule into the cylodextrin cavity 2. dehydration of the organic guest. 3. hydrogen bonding interactions 4. release of the water molecules to bulk water 5. conformational changes or strain release of the Cy. D upon complexation 6. how many water molecules are inside of the cavity before and after complexation. 9

First step: -Cyclodextrine in solution 10

-cyclodextrine 11

Methodology -Born-Oppenheimer Molecular Dynamics -QM/MM calculations -QM : DC-SCC-DFTB* method -MM: employs Rappé’s universal force field (UFF). -Cubic box with a lattice vector length of 34. 92 Å. -1385 water molecules and -Cy. D. -Microcanonical NVE ensemble. -MD run: 160 ps with a time step of 0. 5 fs. -Program: de. Mon program (NRC-2004, Canada) *Zhechkov, L. ; et al. JCTC 2005, 1, 841. * Elstner, et al. , Phys. Rev. B, 1998, 58, 7260. *Porezag, D. et al. Physical Review B 1995, 51, 12947 12

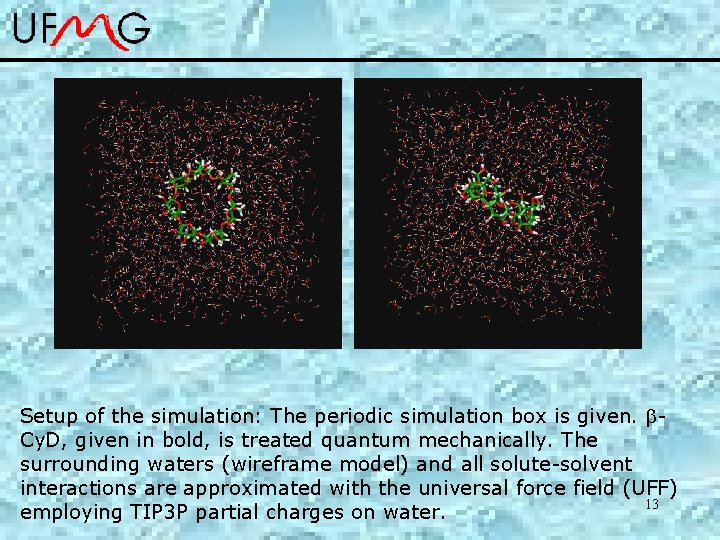
Setup of the simulation: The periodic simulation box is given. Cy. D, given in bold, is treated quantum mechanically. The surrounding waters (wireframe model) and all solute-solvent interactions are approximated with the universal force field (UFF) 13 employing TIP 3 P partial charges on water.

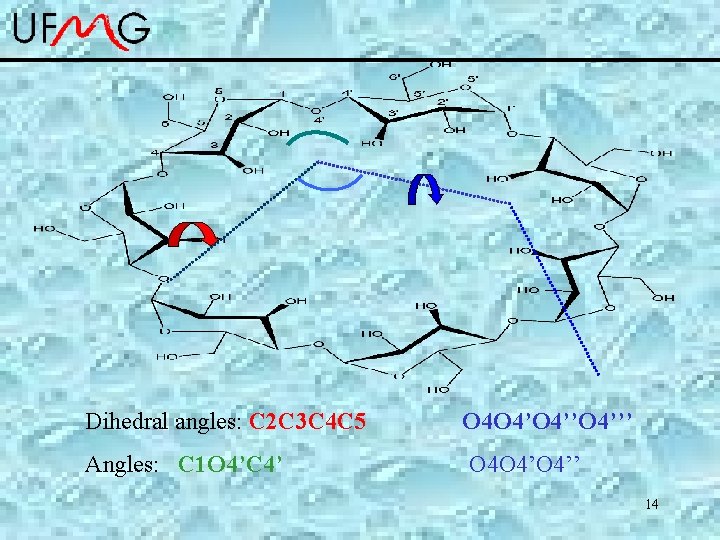
Dihedral angles: C 2 C 3 C 4 C 5 O 4 O 4’’O 4’’’ Angles: C 1 O 4’C 4’ O 4 O 4’’ 14

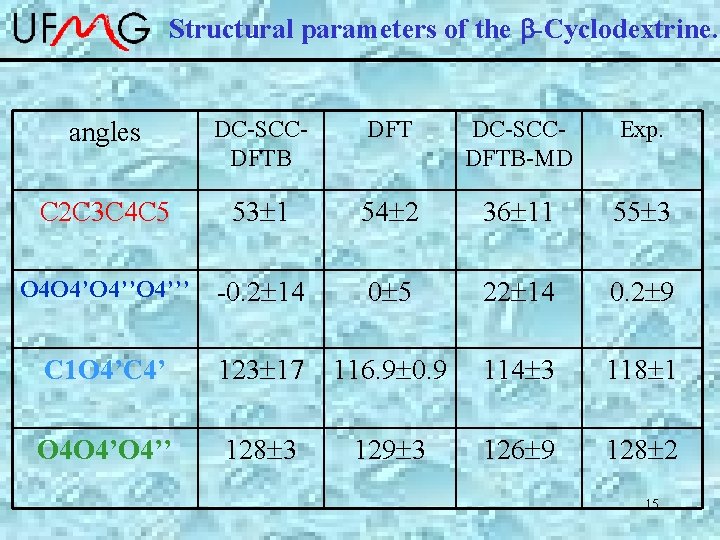
Structural parameters of the b-Cyclodextrine. angles DC-SCCDFTB DFT DC-SCCDFTB-MD Exp. C 2 C 3 C 4 C 5 53 1 54 2 36 11 55 3 O 4 O 4’’O 4’’’ -0. 2 14 0 5 22 14 0. 2 9 C 1 O 4’C 4’ 123 17 116. 9 0. 9 114 3 118 1 O 4 O 4’’ 128 3 129 3 126 9 128 2 15

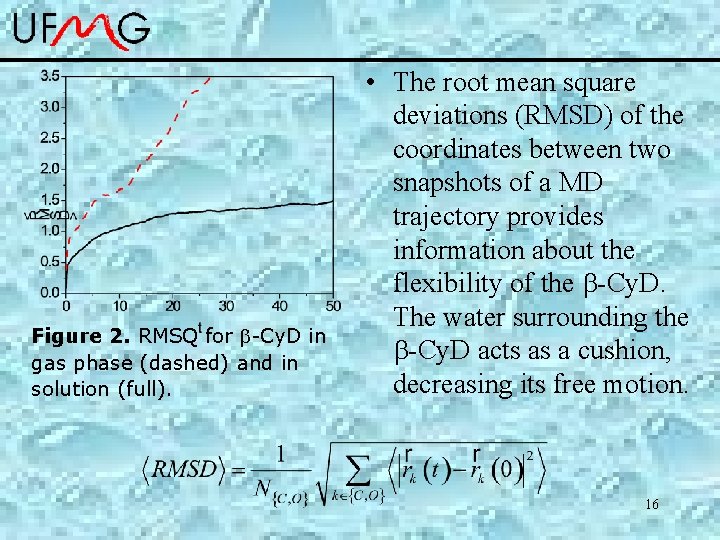
Figure 2. RMSQ for -Cy. D in gas phase (dashed) and in solution (full). • The root mean square deviations (RMSD) of the coordinates between two snapshots of a MD trajectory provides information about the flexibility of the -Cy. D. The water surrounding the -Cy. D acts as a cushion, decreasing its free motion. 16

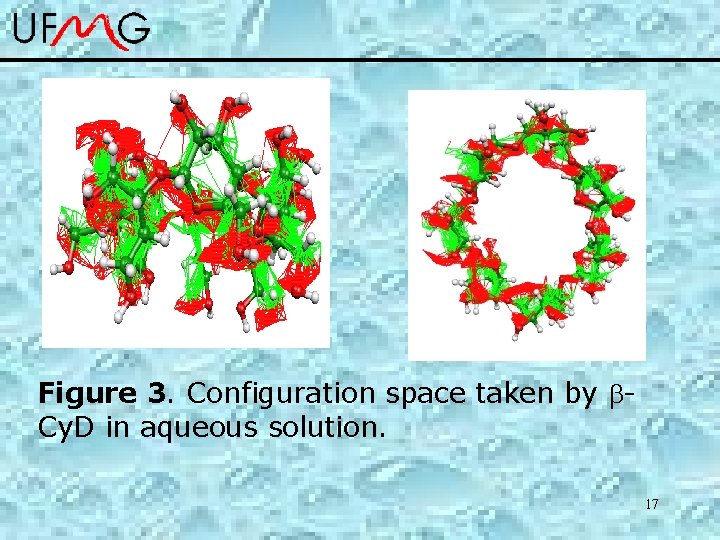
Figure 3. Configuration space taken by Cy. D in aqueous solution. 17

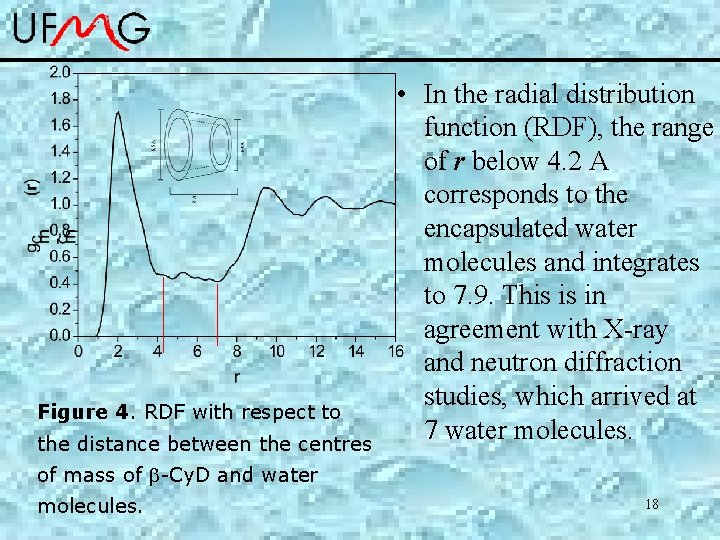
Figure 4. RDF with respect to the distance between the centres • In the radial distribution function (RDF), the range of r below 4. 2 A corresponds to the encapsulated water molecules and integrates to 7. 9. This is in agreement with X-ray and neutron diffraction studies, which arrived at 7 water molecules. of mass of -Cy. D and water molecules. 18

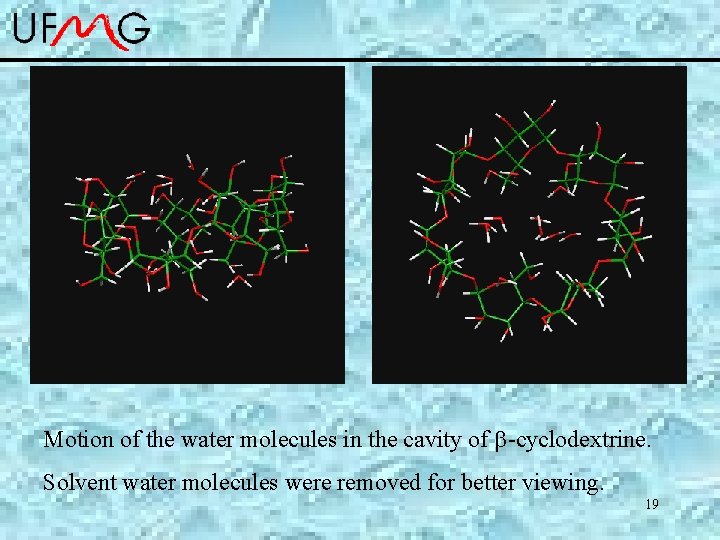
Motion of the water molecules in the cavity of -cyclodextrine. Solvent water molecules were removed for better viewing. 19

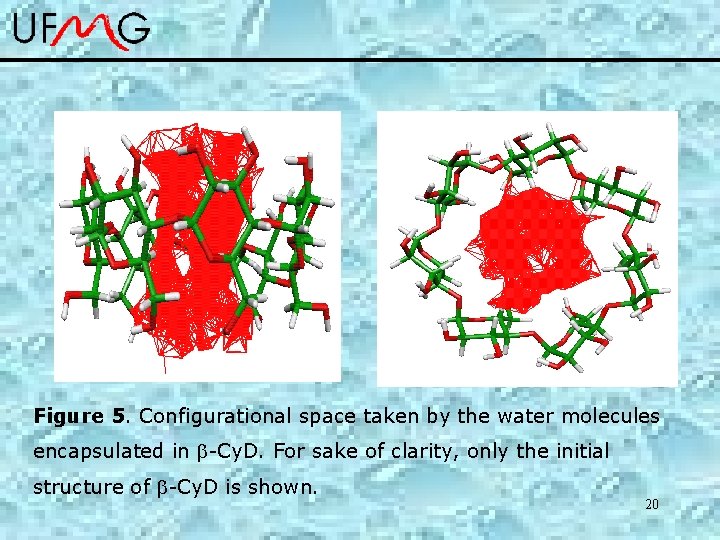
Figure 5. Configurational space taken by the water molecules encapsulated in -Cy. D. For sake of clarity, only the initial structure of -Cy. D is shown. 20

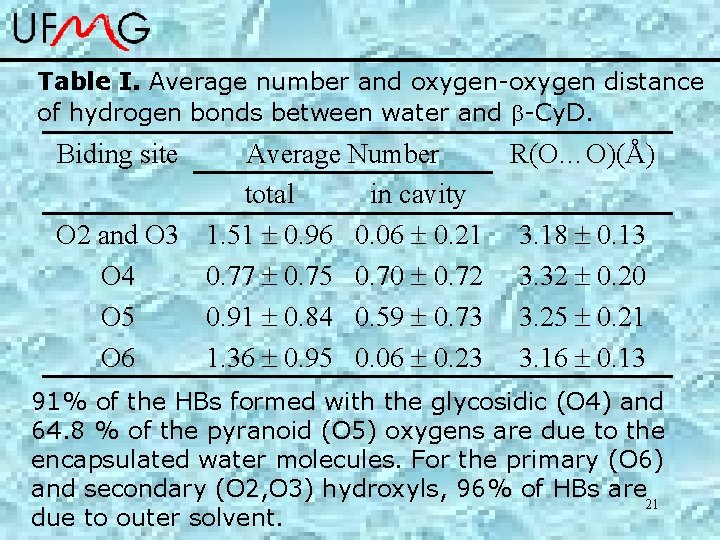
Table I. Average number and oxygen-oxygen distance of hydrogen bonds between water and -Cy. D. Biding site Average Number R(O…O)(Å) total in cavity O 2 and O 3 1. 51 0. 96 0. 06 0. 21 3. 18 0. 13 O 4 0. 77 0. 75 0. 70 0. 72 3. 32 0. 20 O 5 0. 91 0. 84 0. 59 0. 73 3. 25 0. 21 O 6 1. 36 0. 95 0. 06 0. 23 3. 16 0. 13 91% of the HBs formed with the glycosidic (O 4) and 64. 8 % of the pyranoid (O 5) oxygens are due to the encapsulated water molecules. For the primary (O 6) and secondary (O 2, O 3) hydroxyls, 96% of HBs are 21 due to outer solvent.

65% 90% 22

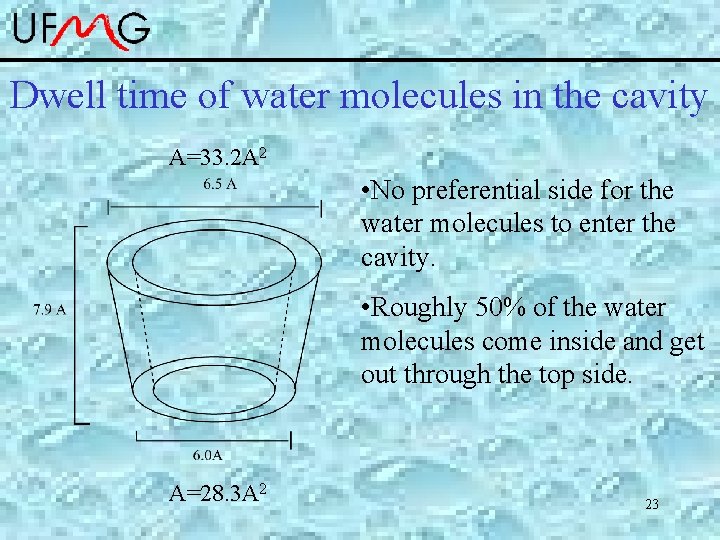
Dwell time of water molecules in the cavity A=33. 2 A 2 • No preferential side for the water molecules to enter the cavity. • Roughly 50% of the water molecules come inside and get out through the top side. A=28. 3 A 2 23

24

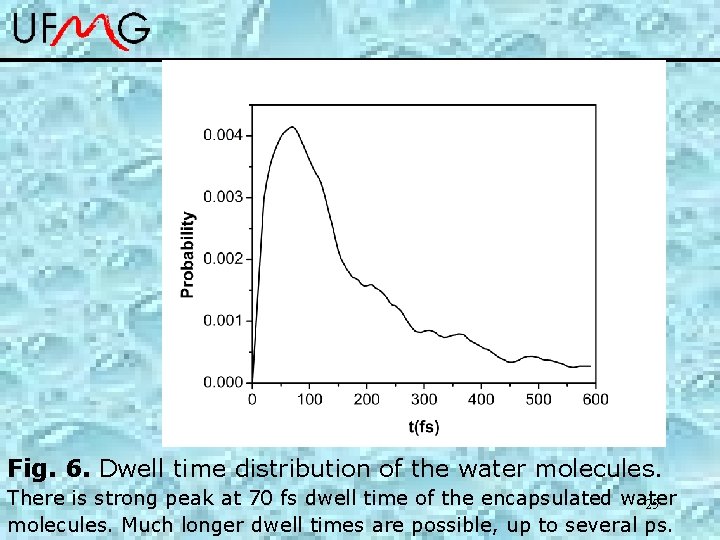
Fig. 6. Dwell time distribution of the water molecules. There is strong peak at 70 fs dwell time of the encapsulated water 25 molecules. Much longer dwell times are possible, up to several ps.

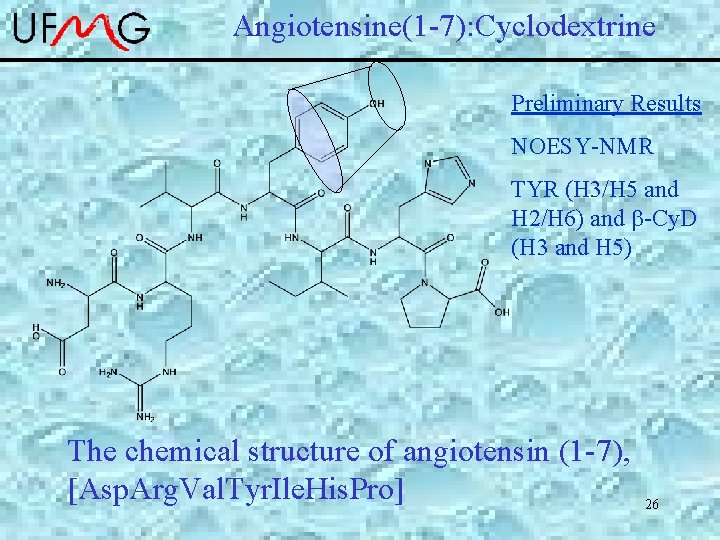
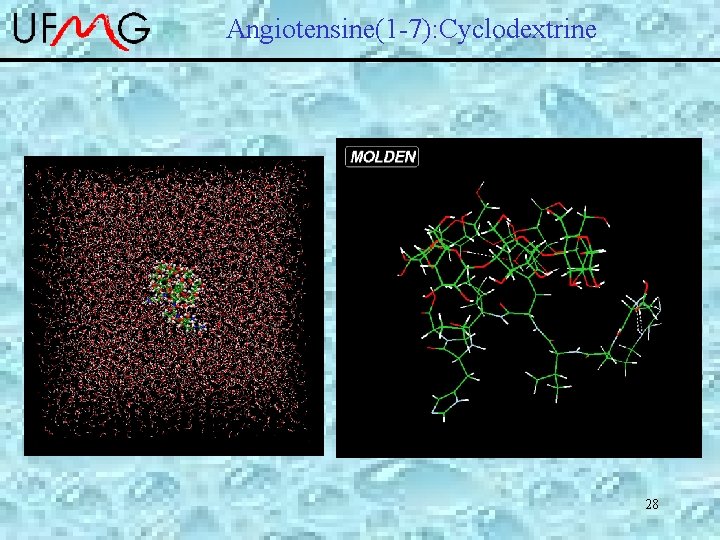
Angiotensine(1 -7): Cyclodextrine Preliminary Results NOESY-NMR TYR (H 3/H 5 and H 2/H 6) and -Cy. D (H 3 and H 5) The chemical structure of angiotensin (1 -7), [Asp. Arg. Val. Tyr. Ile. His. Pro] 26

Methodology -Born-Oppenheimer Molecular Dynamics -QM/MM calculations -QM : DC-DFTB* method -MM: employs Rappé’s universal force field (UFF). -Cubic box with a lattice vector length of 61. 0 Å. -7381 water molecules and Ang(1 -7): -Cy. D. -Microcanonical NVE ensemble. -MD run: with a time step of 0. 5 fs. -Program: de. Mon program (NRC-2004, Canada) *Zhechkov, L. ; et al. JCTC 2005, 1, 841. * Elstner, et al. , Phys. Rev. B, 1998, 58, 7260. *Porezag, D. et al. Physical Review B 1995, 51, 12947 27

Angiotensine(1 -7): Cyclodextrine 28

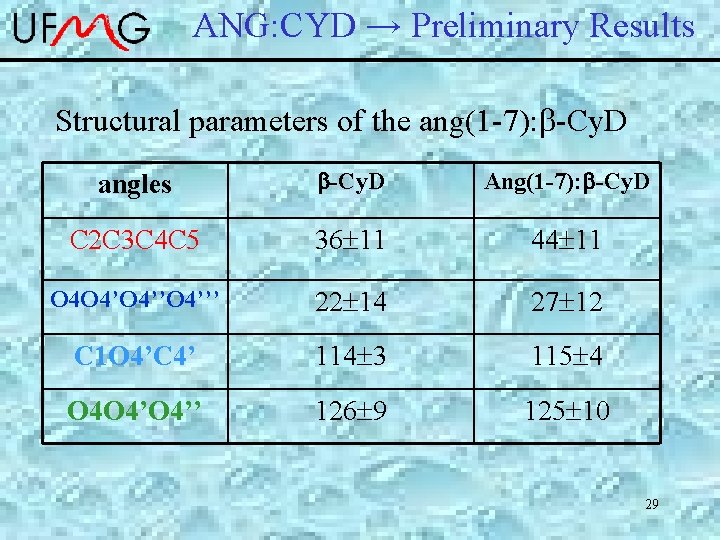
ANG: CYD → Preliminary Results Structural parameters of the ang(1 -7): -Cy. D angles b-Cy. D Ang(1 -7): b-Cy. D C 2 C 3 C 4 C 5 36 11 44 11 O 4 O 4’’O 4’’’ 22 14 27 12 C 1 O 4’C 4’ 114 3 115 4 O 4 O 4’’ 126 9 125 10 29

Water are removed for better view. 30

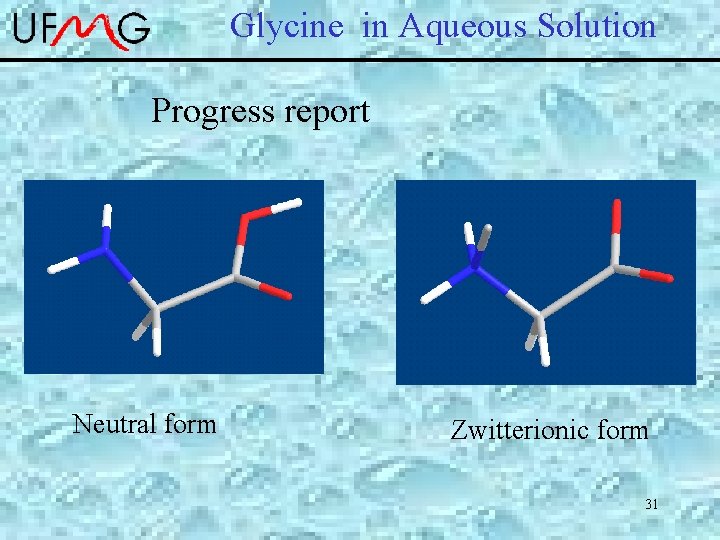
Glycine in Aqueous Solution Progress report Neutral form Zwitterionic form 31

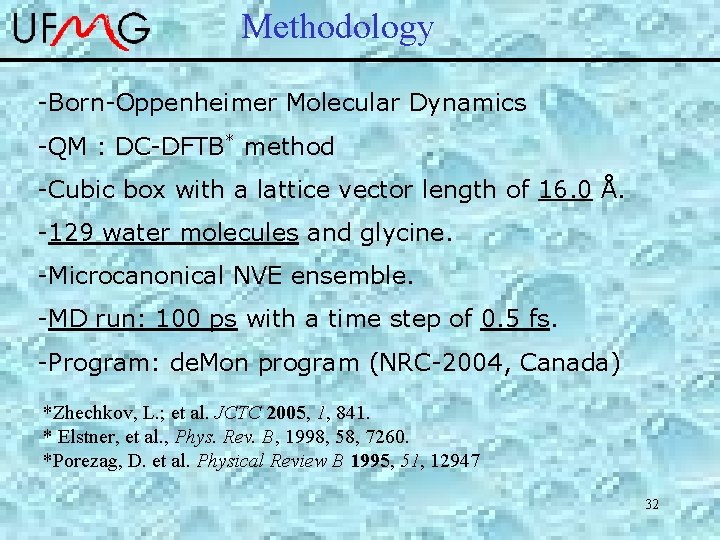
Methodology -Born-Oppenheimer Molecular Dynamics -QM : DC-DFTB* method -Cubic box with a lattice vector length of 16. 0 Å. -129 water molecules and glycine. -Microcanonical NVE ensemble. -MD run: 100 ps with a time step of 0. 5 fs. -Program: de. Mon program (NRC-2004, Canada) *Zhechkov, L. ; et al. JCTC 2005, 1, 841. * Elstner, et al. , Phys. Rev. B, 1998, 58, 7260. *Porezag, D. et al. Physical Review B 1995, 51, 12947 32

33

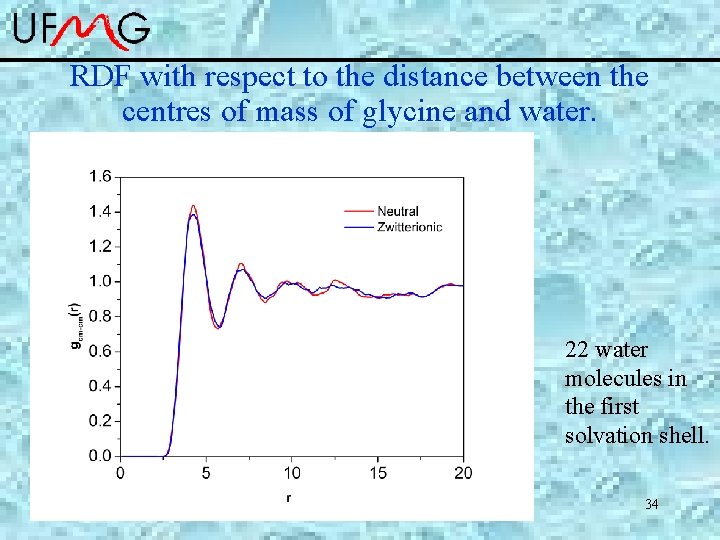
RDF with respect to the distance between the centres of mass of glycine and water. 22 water molecules in the first solvation shell. 34

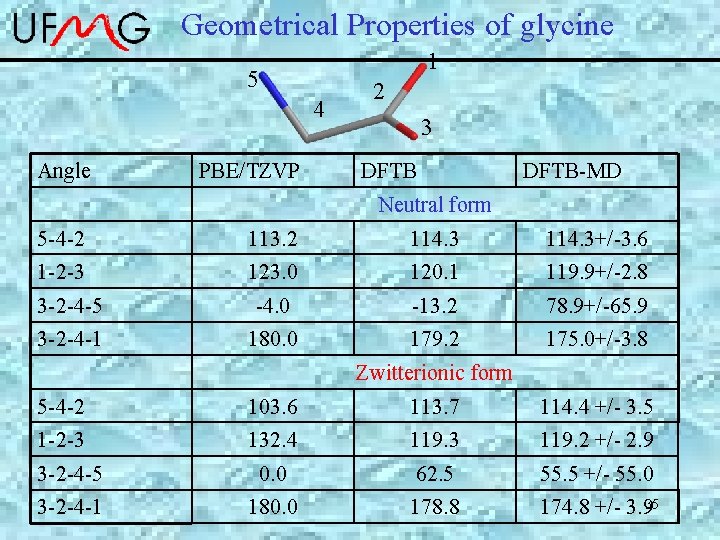
Geometrical Properties of glycine 1 5 4 Angle PBE/TZVP 2 3 DFTB-MD Neutral form 5 -4 -2 113. 2 114. 3+/-3. 6 1 -2 -3 123. 0 120. 1 119. 9+/-2. 8 3 -2 -4 -5 -4. 0 -13. 2 78. 9+/-65. 9 3 -2 -4 -1 180. 0 179. 2 175. 0+/-3. 8 Zwitterionic form 5 -4 -2 103. 6 113. 7 114. 4 +/- 3. 5 1 -2 -3 132. 4 119. 3 119. 2 +/- 2. 9 3 -2 -4 -5 0. 0 62. 5 55. 5 +/- 55. 0 3 -2 -4 -1 180. 0 178. 8 174. 8 +/- 3. 935

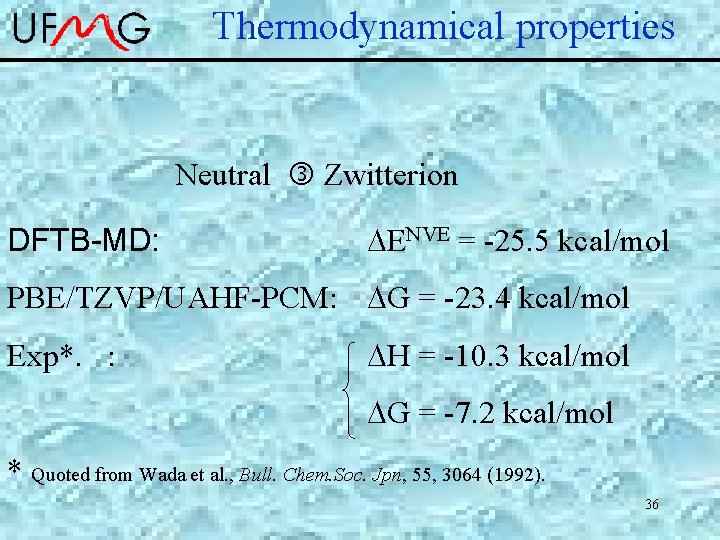
Thermodynamical properties Neutral Zwitterion DFTB-MD: DENVE = -25. 5 kcal/mol PBE/TZVP/UAHF-PCM: DG = -23. 4 kcal/mol Exp*. : DH = -10. 3 kcal/mol DG = -7. 2 kcal/mol * Quoted from Wada et al. , Bull. Chem. Soc. Jpn, 55, 3064 (1992). 36

Grupo de Pesquisa em Química Inorgânica Teórica - GPQIT Collaborators: • Prof. Ruben Sinisterra (DQ-UFMG) • Prof. Hélio F. Dos Santos (DQ-UFJF) • Prof. Gotthard Seifert (TU-Dresden) • Prof. Thomas Heine (TU-Dresden) • Dr. Serguei Patchkovskii (NRC-Canada) 37

Grupo de Pesquisa em Química Inorgânica Teórica - GPQIT Team: • Dr. Heitor Avelino de Abreu (CNPq) • Antonio Noronha (Ph. D Student) • Augusto Faria Oliveira (Ph. D Student) • Luciana Guimarães (Ph. D Student) • Guilherme Ferreira (IC) • Conny Cerai (IC) • Danniel Brandão (IC) • Leonardo R. R. de Oliveira (IC) 38

Support • UFMG • Instituto do Milênio: Água - Uma Visão Mineral(PADCT/CNPq) • CNPq • CAPES • FAPEMIG 39 • PRONEX

40