The What and Who of Advertising Malika Ladha


























- Slides: 29

The ‘What’ and ‘Who’ of Advertising Malika Ladha malikal@paab. ca PAAB Reviewer

Chester Bowles “ 20% of the regulated population will automatically comply with any regulation, 5% will attempt to evade it, and 75% will comply so long as they think the 5% will be caught and punished. ” Regulator and member in the 1941 U. S. Wartime Office of Price Administration

CANADA Self-regulation Government Regulation

For success Three critical elements: • An effective mechanism (preclearance is best) • Support from major industry players • Support and trust from the government Self regulation is like a vaccine that prevents bad things from happening

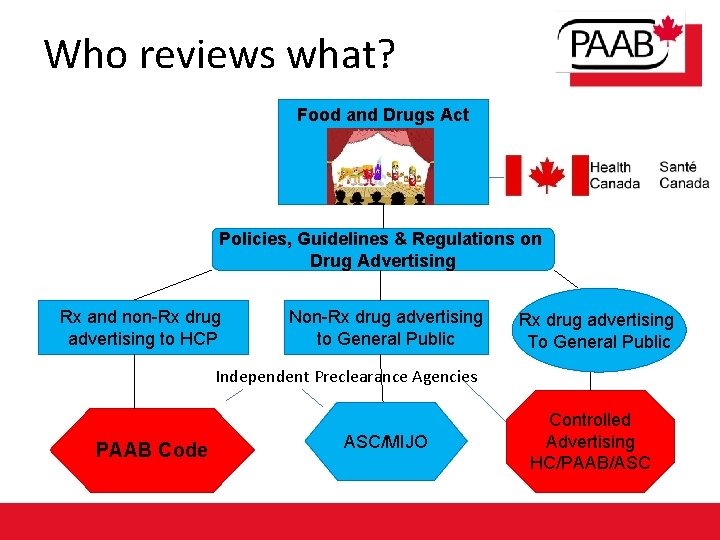
Who reviews what? Food and Drugs Act Health Canada Policies, Guidelines & Regulations on Drug Advertising Rx and non-Rx drug advertising to HCP Non-Rx drug advertising to General Public Rx drug advertising To General Public Independent Preclearance Agencies PAAB Code ASC/MIJO Controlled Advertising HC/PAAB/ASC

Important takeaways üDrug advertising is regulated • No person shall. . . advertise a new drug unless…the Minister has issued a Notice of Compliance to the manufacturer of the new drug… (FDA c. 08. 002) • No person shall. . . advertise any drug in a manner that is false, misleading or deceptive or is likely to create an erroneous impression regarding its character, value, quantity, composition, merit or safety. (section 9. (1)) üSelf-regulation is a privilege, not a right üIndustry’s actions must be aligned with the regulations to promote credibility and trust through improvement of patient care

What is Drug Advertising? Definition in section 2 of the Food & Drugs Act: “Any representation by any means whatever for the purpose of promoting directly or indirectly the sale or disposal of any food, drug, cosmetic or device”

Food and Drugs Act Section 9(1) No person shall label, package, treat, process, sell or advertise any drug in a manner that is false, misleading or deceptive or is likely to create an erroneous impression regarding its composition, merit or safety

The Distinction Between Advertising and Other Activities • What is the context in which the message is disseminated ? • Who are the primary and secondary audiences ? • Who delivers the message (the provider) ? • Who sponsors the message and how ? • What influence does the drug manufacturer have on the message content ? • What is the content of the message ? • With what frequency is the message delivered ?

The Distinction Between Advertising and Other Activities: “No one factor in itself will determine whether or not a particular message is advertising. ” …If uncertain, don’t hesitate to ask PAAB. We’ll respond to requests for written opinions within 4 days.

PAAB

PAAB Brief History • • Incorporated 1976 Government threat to industry Multi-stakeholder approach Unique model Code applies to all companies Dynamic code Evolving organization Between Industry and Government 12

PAAB VISION – Trusted healthcare product communication that promotes optimal health MISSION – To provide a preclearance review that fosters trustworthy healthcare communications within the regulatory framework. VALUES – Integrity, Competency, Credibility, Independence, Excellence, Transparency

MANDATE • The PAAB is an independent review agency whose primary role is to ensure that healthcare product communication for prescription, non-prescription, biological and natural health products is accurate, balanced and evidence-based, and reflects current and best practice. • The PAAB also monitors trends in health product advertising and promotion and adjusts its code and practices as required to fulfill its mandate.

Scope • The scope of the PAAB includes promotional healthcare product communication for prescription, non-prescription, biological and natural health products to health care professionals in all media. • PAAB also provides advisory comments on directto-consumer materials for prescription drugs. PAAB’s Scope evolves with the regulatory framework.

New PAAB Code was implemented on July 1, 2013

PAAB Code of Advertising Acceptance • Dynamic, reflects current marketplace • Works in best interest of patients • Requires 2/3 majority vote of members to revise • Standards including: – regulatory – scientific – clinical – ethical principles

PAAB’s Board of Directors • pharmaceutical trade associations – Rx&D, CGPA, CHPC, Biote. Canada • health professionals - CMA, CPh. A, FMOQ, AFMC • patients - Best Medicines Coalition (BMC) CARP, Consumers Council of Canada (CCC) • Can Assoc of Medical Publishers (CAMP) • advertising industry (AMAA) • Chair, Vice-Chair, Treasurer

• Health Canada is an ex-officio observer and advisor “without relinquishing authority under the Food and Drugs Act” • PAAB Commissioner liaison with Manager, Advertising and Risk Communications Section, Marketed Health Products Directorate • Annual Bilateral Consultation meetings • Policy - Roles and Consultation Related to Advertising Review, Health Canada and preclearance agencies

PAAB preclearance services PAAB code covers • HCP Advertising • Patient Information provided through HCPs PAAB advisory service (using Health Canada policy documents): • Consumer Information • Consumer Advertising Health Canada and Advertising Preclearance Agencies’ Roles Related to Health Product Advertising: http: //www. hc-sc. gc. ca/dhp-mps/advert-publicit/pol/role_apa-pca-eng. php

PAAB Code section 6. 6 à Exemptions from PAAB review PAAB code 6. 6(iv): Use of drug name only in a context not linked to therapeutic or promotional messages, other than those listed below, in any way. Examples: – Company price lists containing no therapeutic claims, price comparisons or claims of company or product merit, status or issues – Message comprised only of the words “now on provincial formulary” (or equivalent) in a manner which is not linked to a therapeutic message in any way – Message of “Available at company X” – A message of “Congratulations to company X on their 30 th anniversary – sponsored by Company X makers of product Y” – Packshots if no therapeutic claims are visible

Target your message to match your audience

Three regulatory audiences: HCP: Messaging directed to licensed members of the professions of medicine, dentistry, naturopathy, nursing, pharmacy and related health disciplines and institutions. Patient: Messaging directed to individuals prescribed that product OR messaging in a tool intended for use by HCPs only during counseling. Consumer: Messaging directed to the general public. Readily accessible by individuals who have not been prescribed the product.

APS directed towards PATIENTS

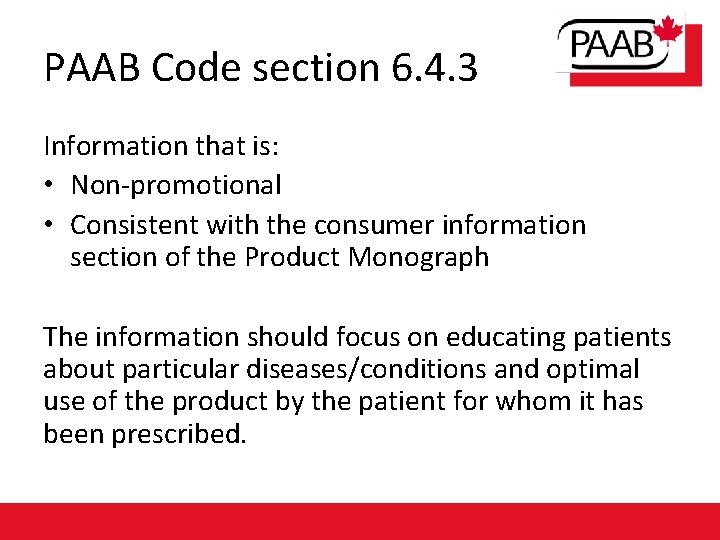
PAAB Code section 6. 4. 3 Information that is: • Non-promotional • Consistent with the consumer information section of the Product Monograph The information should focus on educating patients about particular diseases/conditions and optimal use of the product by the patient for whom it has been prescribed.

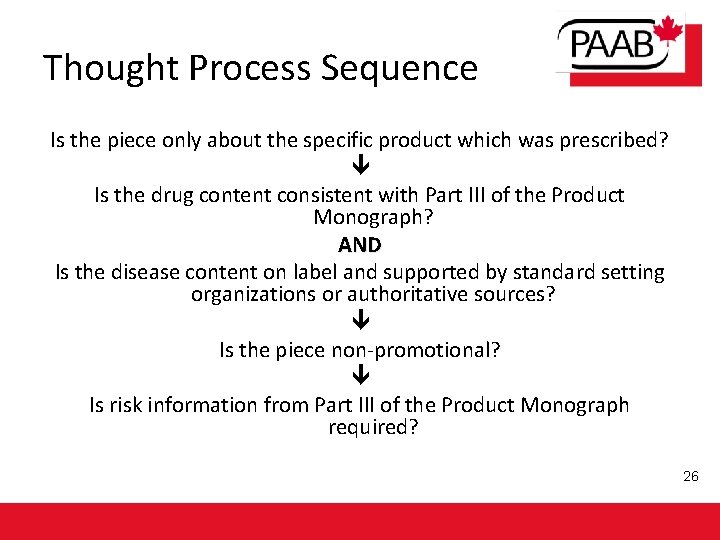
Thought Process Sequence Is the piece only about the specific product which was prescribed? Is the drug content consistent with Part III of the Product Monograph? AND Is the disease content on label and supported by standard setting organizations or authoritative sources? Is the piece non-promotional? Is risk information from Part III of the Product Monograph required? 26

Standard Setting Organizations What they are: • Group generally considered to be a credible source for patient information • Package complex medical information in a manner which is easy for patients to understand What they aren't: • A person • A commercial website • Controlled by pharma 27

Standard Setting Organizations Examples: • Patient groups (e. g. , the Asthma Society of Canada) • Medical institutions (e. g. , a hospital) • Health care professional organizations (e. g. , Canadian Nurses Association) • Consensus groups (e. g. , Canadian Diabetes Association) 28

Questions?
 Paab code
Paab code Rehana ladha
Rehana ladha Rehana ladha
Rehana ladha Global advertising and international advertising
Global advertising and international advertising Malika meddahi
Malika meddahi Malika kalam
Malika kalam Malika meddahi
Malika meddahi Malika kishwar
Malika kishwar Raouf oufkir
Raouf oufkir Anatomy of an abstract
Anatomy of an abstract Malika pritchett
Malika pritchett Malika roman isler
Malika roman isler Soraya malika
Soraya malika Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống