The Water Molecule Line Position and Line Intensity







- Slides: 33

The Water Molecule: Line Position and Line Intensity Analyses up to the Second Triad L. H. Coudert, a G. Wagner, b M. Birk, b and J. -M. Flauda a. Laboratoire b. Deutsches Interuniversitaire des Systèmes Atmosphériques, France Zentrum für Luft- und Raumfahrt e. V. , Institut für Methodik der Fernerkundung, Germany

Water facts Water is one of the most studied molecules In the Journal of Molecular Spectroscopy, 16 articles were published since June 2005 including: 15 experimental papers and 1 theoretical paper A theoretician has a lot of work to do just fitting these data

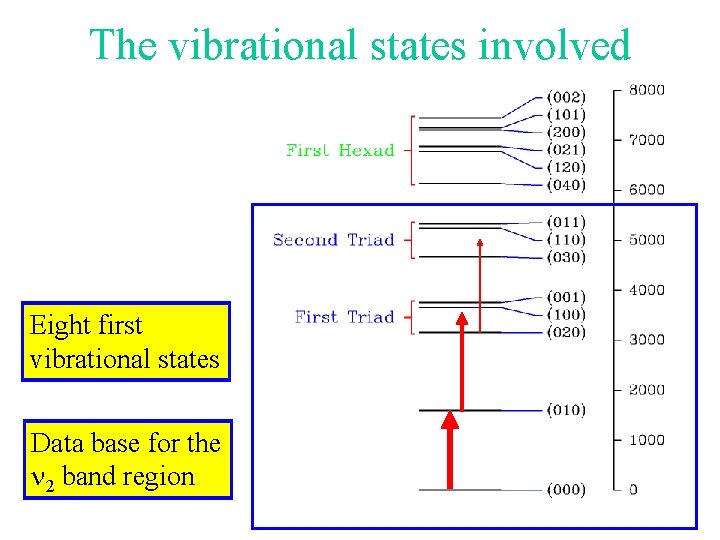
The vibrational states involved Eight first vibrational states Data base for the 2 band region

Overview • The theoretical approach • The line position analysis • The data set • Results • The line strength analysis • The spectroscopic parameters • The new measurements • Results • Building a new data base for water

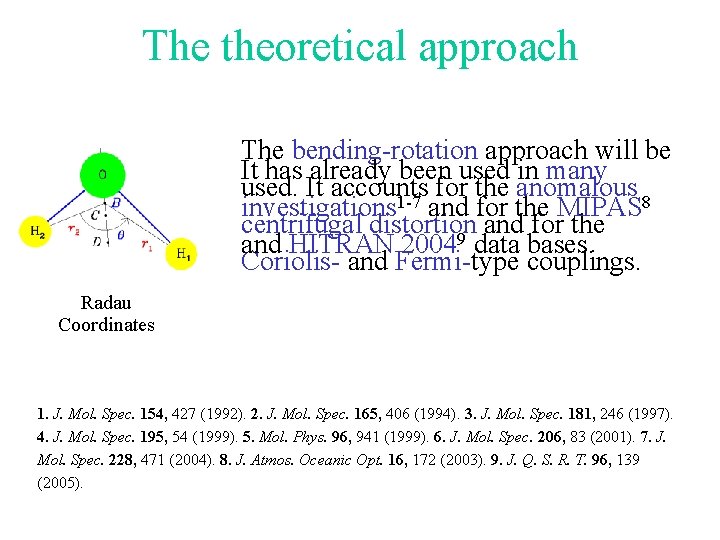
The theoretical approach The bending-rotation approach will be It has already been used in many used. It accounts for the anomalous 8 1 -7 investigations and for the MIPAS centrifugal distortion and for the 9 and HITRAN 2004 data bases. Coriolis- and Fermi-type couplings. Radau Coordinates 1. J. Mol. Spec. 154, 427 (1992). 2. J. Mol. Spec. 165, 406 (1994). 3. J. Mol. Spec. 181, 246 (1997). 4. J. Mol. Spec. 195, 54 (1999). 5. Mol. Phys. 96, 941 (1999). 6. J. Mol. Spec. 206, 83 (2001). 7. J. Mol. Spec. 228, 471 (2004). 8. J. Atmos. Oceanic Opt. 16, 172 (2003). 9. J. Q. S. R. T. 96, 139 (2005).

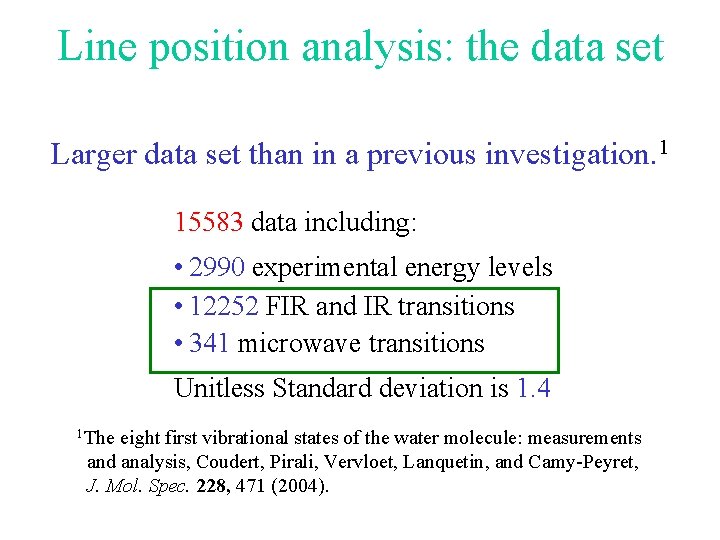
Line position analysis: the data set Larger data set than in a previous investigation. 1 15583 data including: • 2990 experimental energy levels • 12252 FIR and IR transitions • 341 microwave transitions Unitless Standard deviation is 1. 4 1 The eight first vibrational states of the water molecule: measurements and analysis, Coudert, Pirali, Vervloet, Lanquetin, and Camy-Peyret, J. Mol. Spec. 228, 471 (2004).

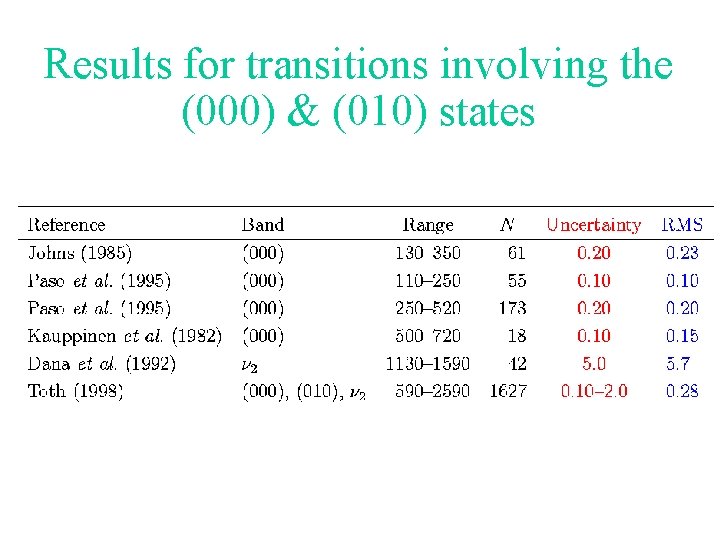
Results for transitions involving the (000) & (010) states

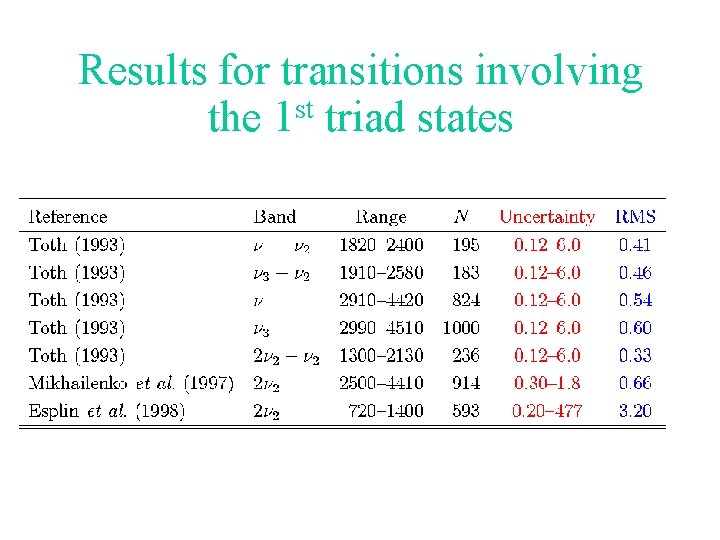
Results for transitions involving the 1 st triad states

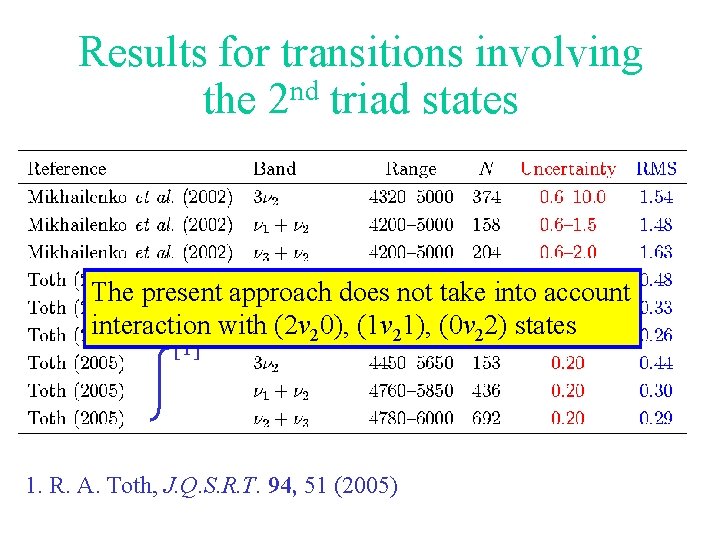
Results for transitions involving the 2 nd triad states The present approach does not take into account interaction with (2 v 20), (1 v 21), (0 v 22) states [1] 1. R. A. Toth, J. Q. S. R. T. 94, 51 (2005)

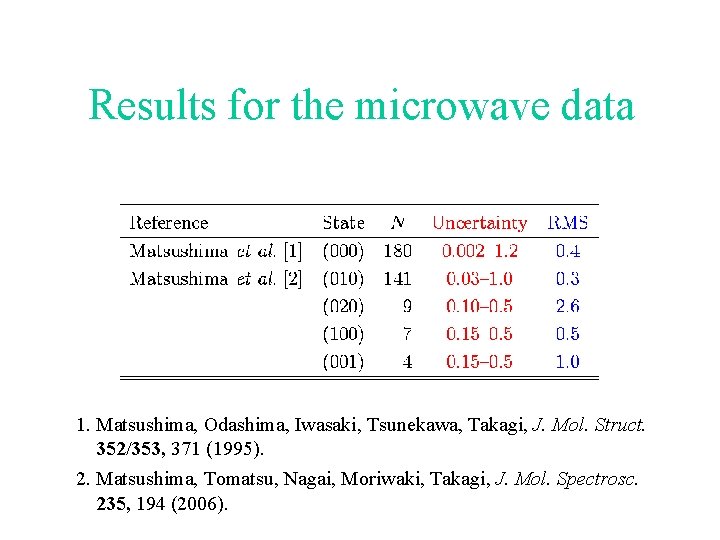
Results for the microwave data 1. Matsushima, Odashima, Iwasaki, Tsunekawa, Takagi, J. Mol. Struct. 352/353, 371 (1995). 2. Matsushima, Tomatsu, Nagai, Moriwaki, Takagi, J. Mol. Spectrosc. 235, 194 (2006).

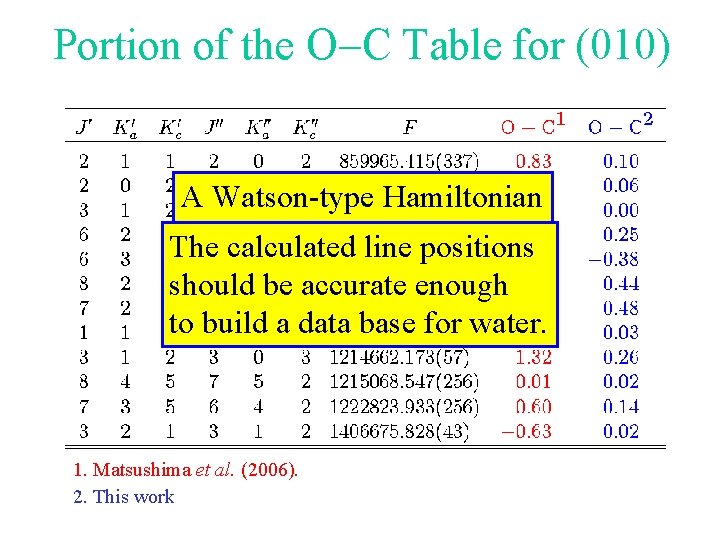
Portion of the O-C Table for (010) A Watson-type Hamiltonian cannot be usedline for positions water. The calculated should be accurate enough to build a data base for water. 1. Matsushima et al. (2006). 2. This work

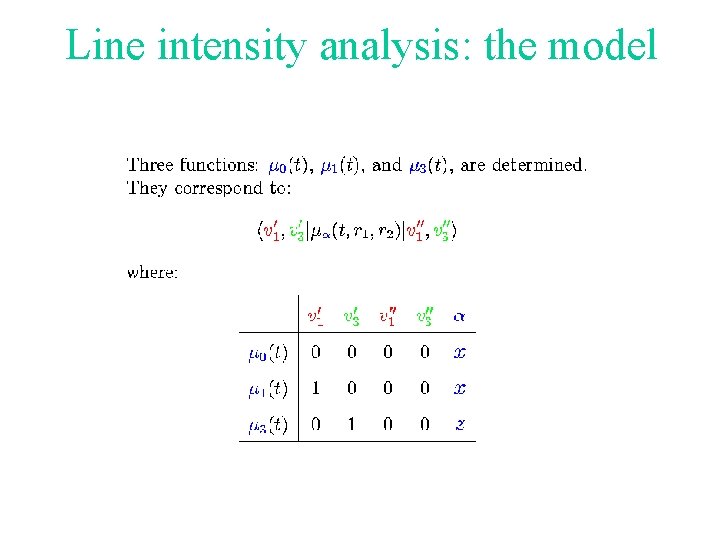
Line intensity analysis: the model

The new measurements • Toth, J. Q. S. R. T. 94, 51 (2005) 3 2, 1+ 2, 3+ 2, 3 2 - 2, 1+ 2 - 2, 3+ 2 - 2 1559 transitions • This work 2, 2 2 - 2 879 transitions

Experimental • • Instrument: Bruker IFS 120 HR Sample cells: Short cell, White cell Wavenumber range 1250 -1750 cm-1 MOPD: 187. 5 cm Optical path evacuated Detector: Photoconductive MCT Temperature measurement: Calibrated Pt 100 + Lakeshore temperature controller • Pressure measurement: Thermostated, calibrated MKS Baratrons: 1, 100, 1000, 5000 mb More details see presentation by Georg Wagner: „Water Pressure Broadening: A Never-ending Story“

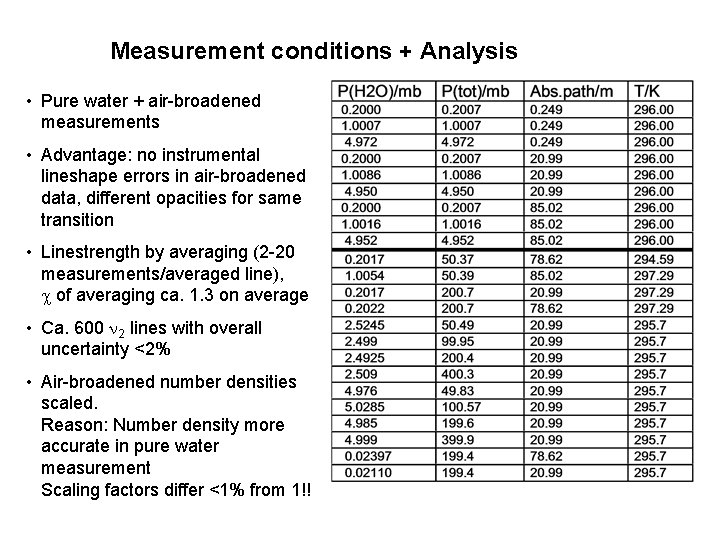
Measurement conditions + Analysis • Pure water + air-broadened measurements • Advantage: no instrumental lineshape errors in air-broadened data, different opacities for same transition • Linestrength by averaging (2 -20 measurements/averaged line), of averaging ca. 1. 3 on average • Ca. 600 2 lines with overall uncertainty <2% • Air-broadened number densities scaled. Reason: Number density more accurate in pure water measurement Scaling factors differ <1% from 1!!

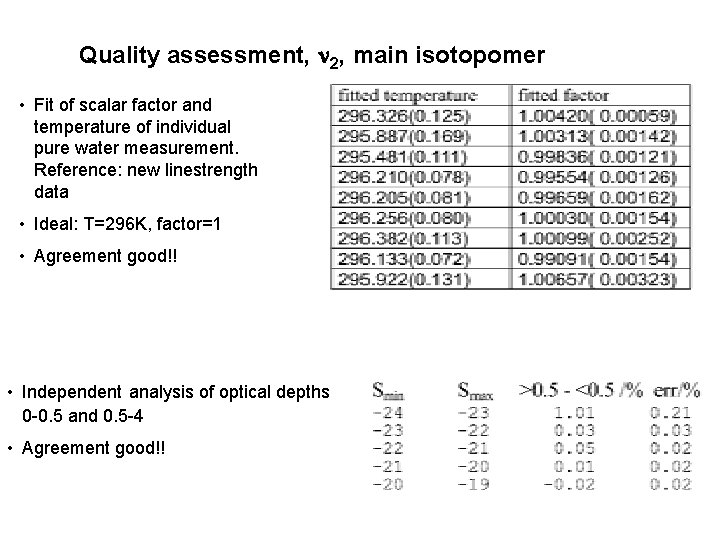
Quality assessment, 2, main isotopomer • Fit of scalar factor and temperature of individual pure water measurement. Reference: new linestrength data • Ideal: T=296 K, factor=1 • Agreement good!! • Independent analysis of optical depths 0 -0. 5 and 0. 5 -4 • Agreement good!!

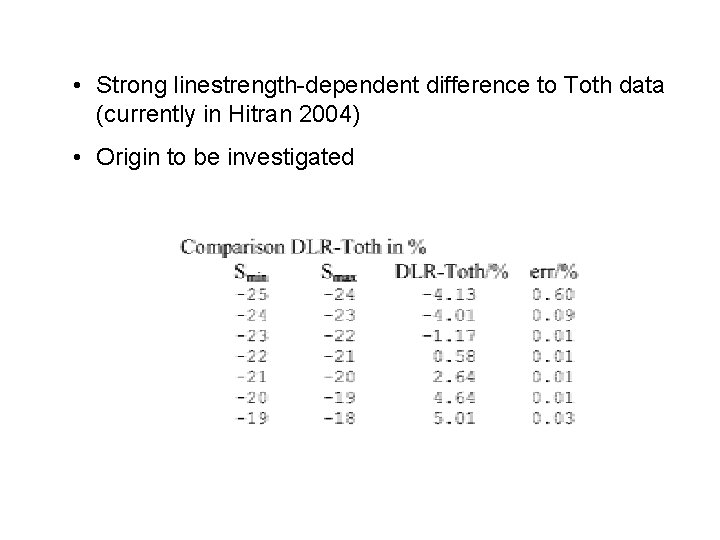
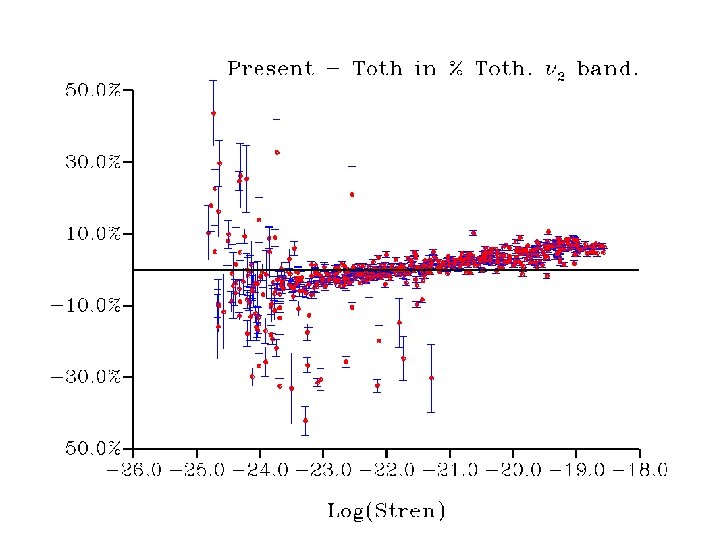
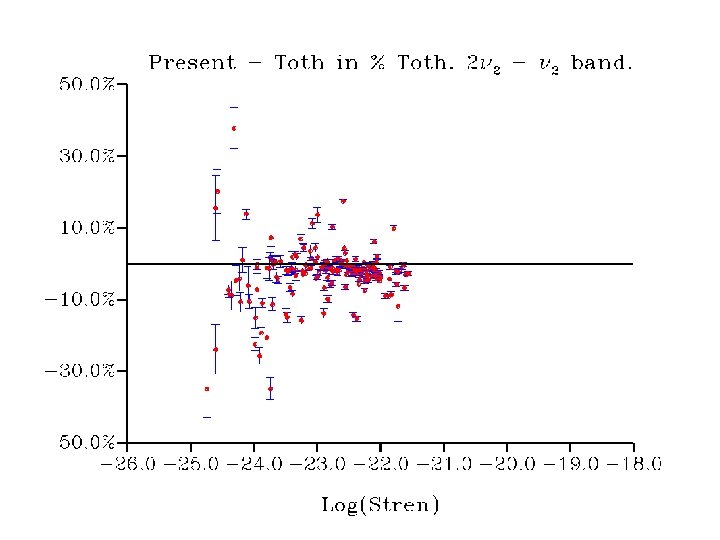
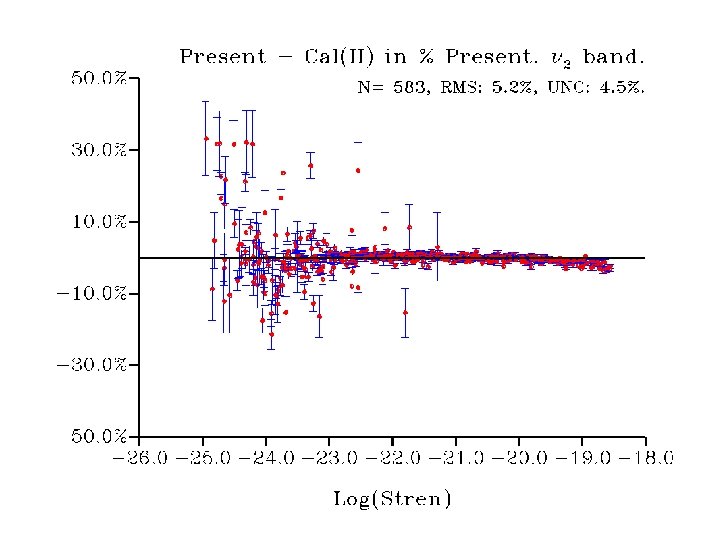
• Strong linestrength-dependent difference to Toth data (currently in Hitran 2004) • Origin to be investigated



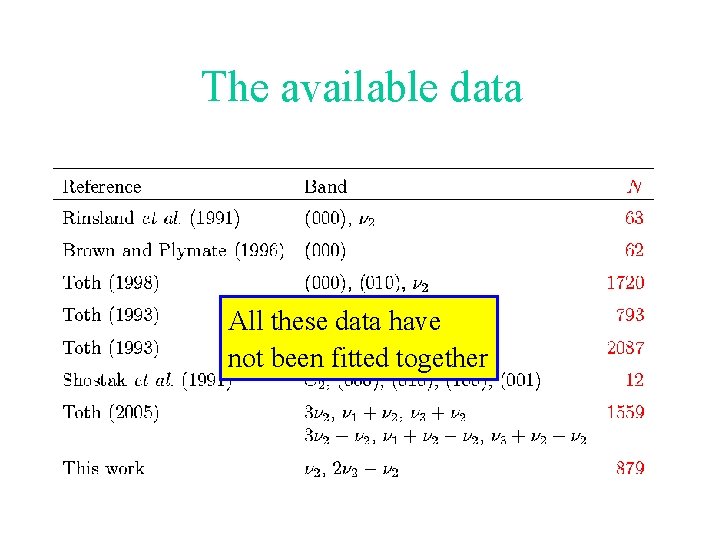
The available data All these data have not been fitted together

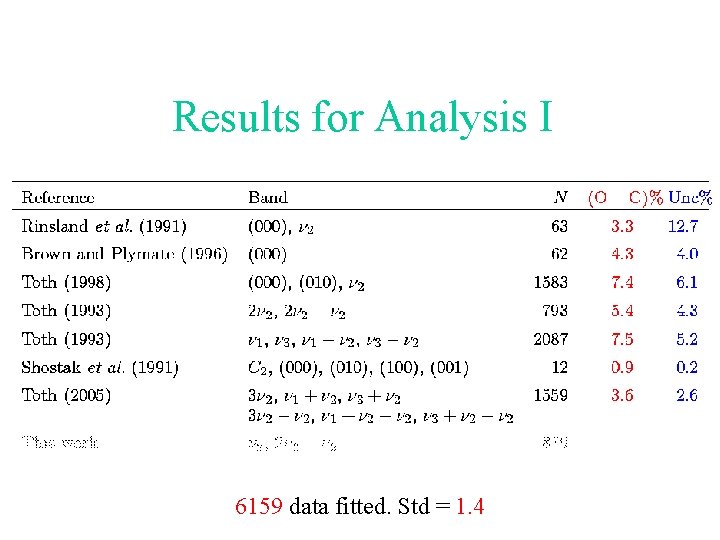
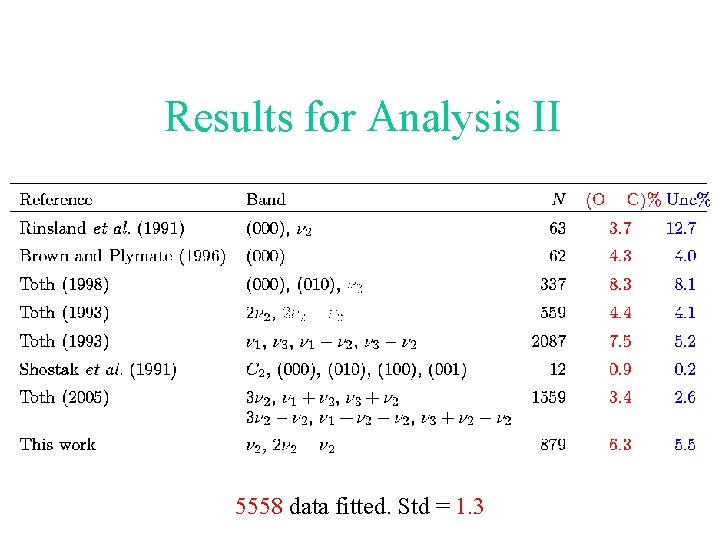
The two line strength analyses Analysis I: The previous data except the measurements carried out in this work. Analysis II: The previous data except Toth’s measurements for transitions involving the 2 and 2 2 - 2 bands.

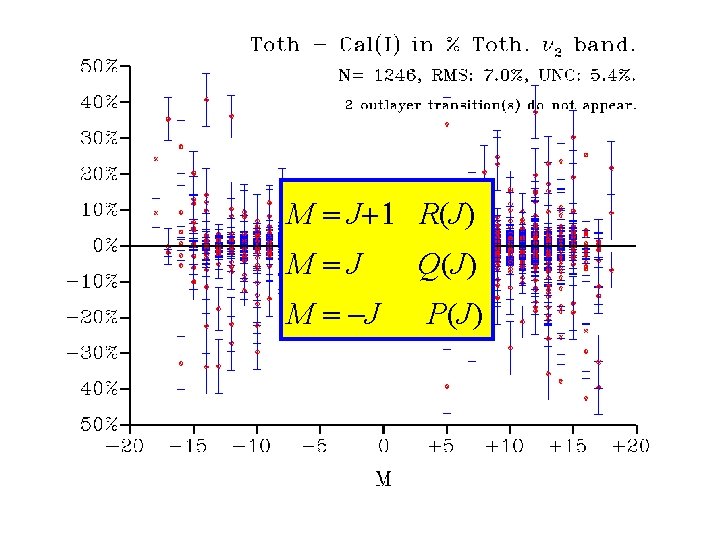
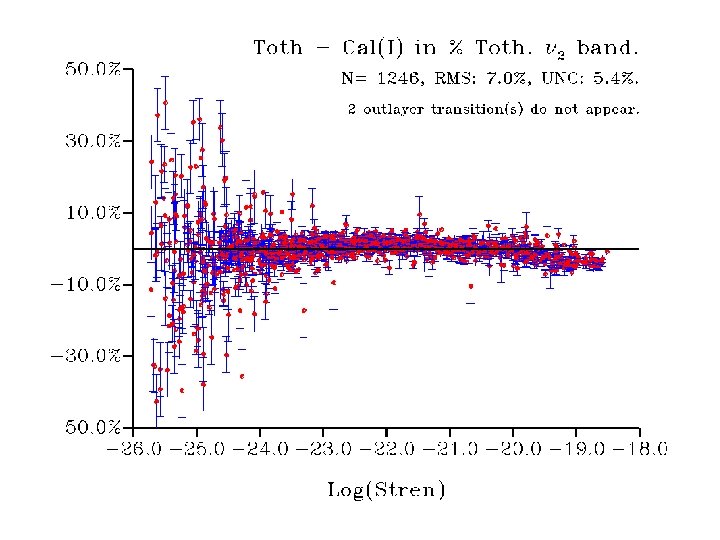
Results for Analysis I 6159 data fitted. Std = 1. 4

M = J+1 R(J) AA M=J Q(J) M = -J P(J)


Results for Analysis II 5558 data fitted. Std = 1. 3



Calculated with Hitran 04

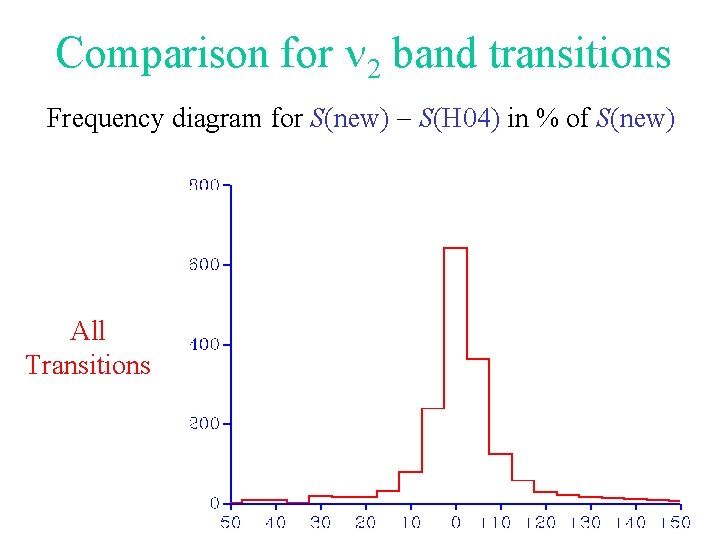
Can we improve Hitran 04 ? • Using the results of Analysis II, a data base was built. • This new data base was compared with Hitran 04.

Comparison for 2 band transitions Frequency diagram for S(new) - S(H 04) in % of S(new) All Transitions

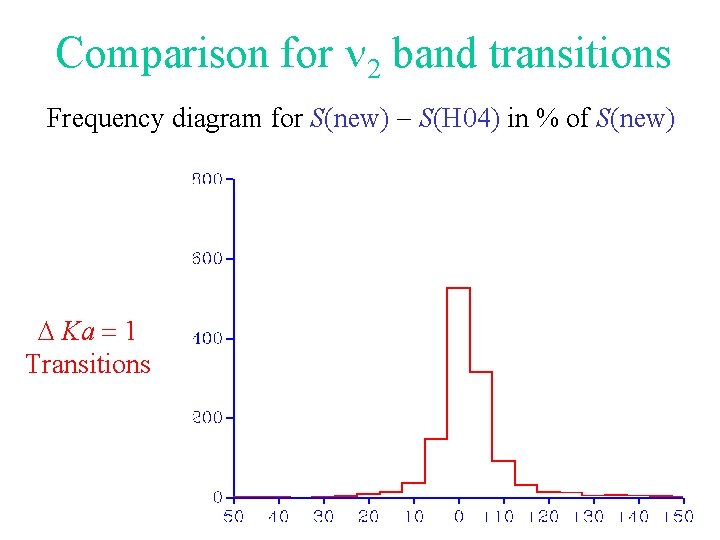
Comparison for 2 band transitions Frequency diagram for S(new) - S(H 04) in % of S(new) D Ka = 1 Transitions

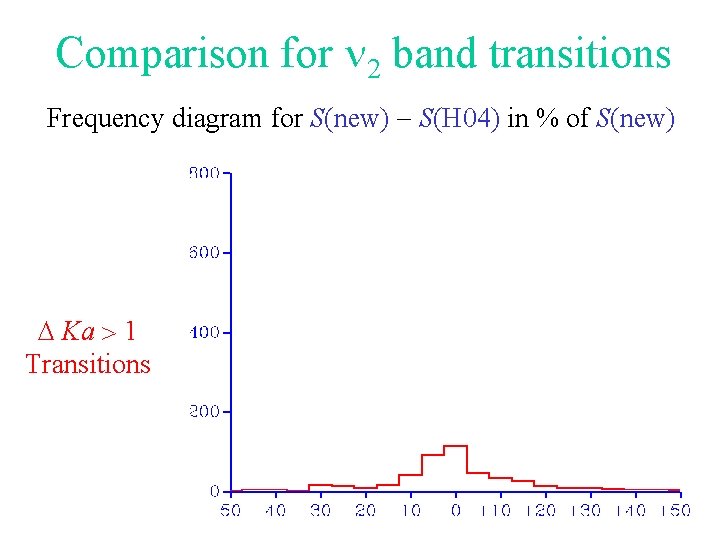
Comparison for 2 band transitions Frequency diagram for S(new) - S(H 04) in % of S(new) D Ka > 1 Transitions

Future work