The Vascular Quality Initiative Using PSO Registry Data




























- Slides: 75

The Vascular Quality Initiative: Using PSO Registry Data to Improve Care Adam W. Beck, M. D. , FACS Chair, Arterial Quality Council Society for Vascular Surgery Patient Safety Organization

Disclosures • No relevant disclosures

Launched by Society for Vascular Surgery in 2011 • Mission: To improve the quality, safety, effectiveness and cost of vascular health care by collecting and exchanging information. • 3 Components: – National Registries in a Patient Safety Organization – Regional Quality Improvement Groups • Based on Vascular Study Group of New England, 2002 – Web-based data collection - reporting system

Patient Safety Organization (Patient Safety Act) • Allows patient identified information to be collected for quality improvement without informed consent • Protects work product (any comparative data) from discovery to encourage honest reporting • Precludes comparative data to be used for physician disciplinary purposes or marketing • Allows non-identifiable data to be published – Statistical de-identification of patient, provider, hospital • Ideal vehicle for quality improvement registry

National Registries in a Patient Safety Organization • Carotid disease – Endarterectomy and stenting • Aortic disease – Open and endovascular abdominal aneurysm repair – Endovascular repair thoracic aorta • Lower extremity arterial disease – Bypass, interventional procedures, amputation • • Medical Management (in development) Dialysis access Vena cava filters Varicose veins

Advantages of SVS PSO Registry Data • Allows data from all patients to be included – Not biased by those who only give consent • Much more detailed information than claims data – Pre-, intra-, and post-op variables (> 150 per procedure) • One year follow-up for key outcomes – Completed in physician’s office • All consecutive procedures – allows rate calculation – Audited against hospital and physician claims data • Longer follow-up with matched Medicare Claims – Survival also from Social Security Death Index

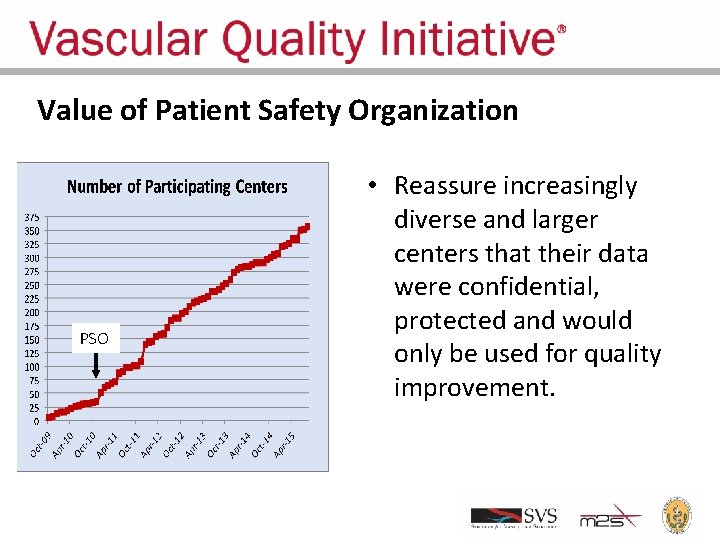
Value of Patient Safety Organization PSO • Reassure increasingly diverse and larger centers that their data were confidential, protected and would only be used for quality improvement.

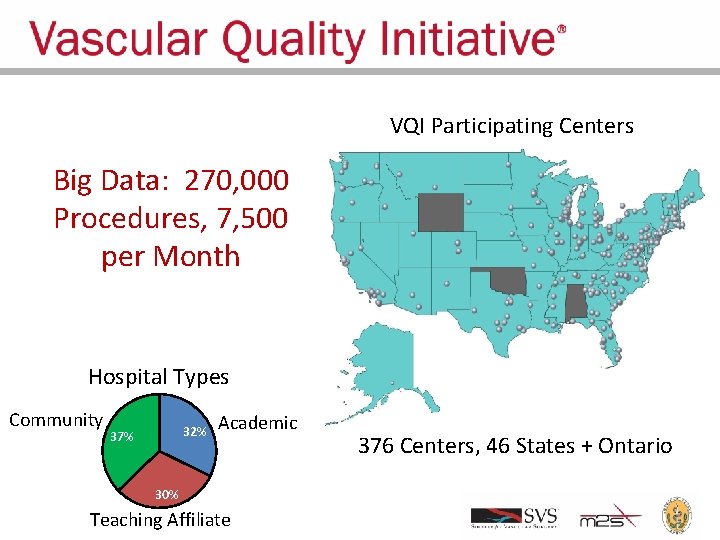
VQI Participating Centers Big Data: 270, 000 Procedures, 7, 500 per Month Hospital Types Community 32% 37% Academic 30% Teaching Affiliate 376 Centers, 46 States + Ontario

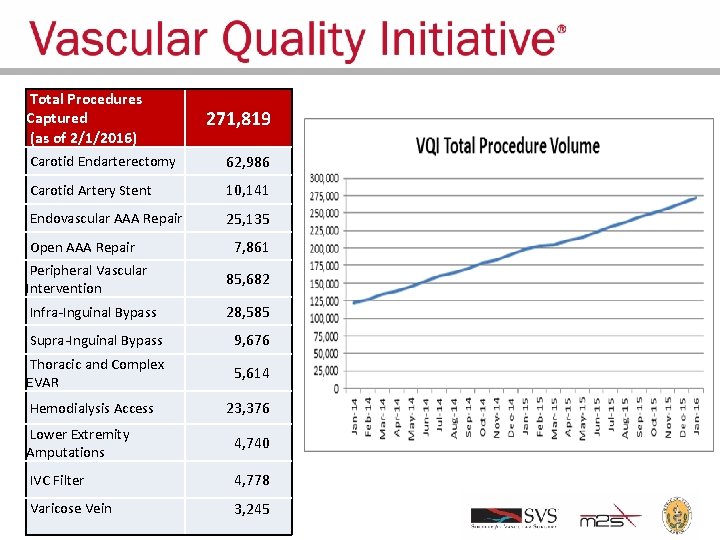
Total Procedures Captured (as of 2/1/2016) 271, 819 Carotid Endarterectomy 62, 986 Carotid Artery Stent 10, 141 Endovascular AAA Repair 25, 135 Open AAA Repair 7, 861 Peripheral Vascular Intervention 85, 682 Infra-Inguinal Bypass 28, 585 Supra-Inguinal Bypass 9, 676 Thoracic and Complex EVAR 5, 614 Hemodialysis Access 23, 376 Lower Extremity Amputations 4, 740 IVC Filter 4, 778 Varicose Vein 3, 245

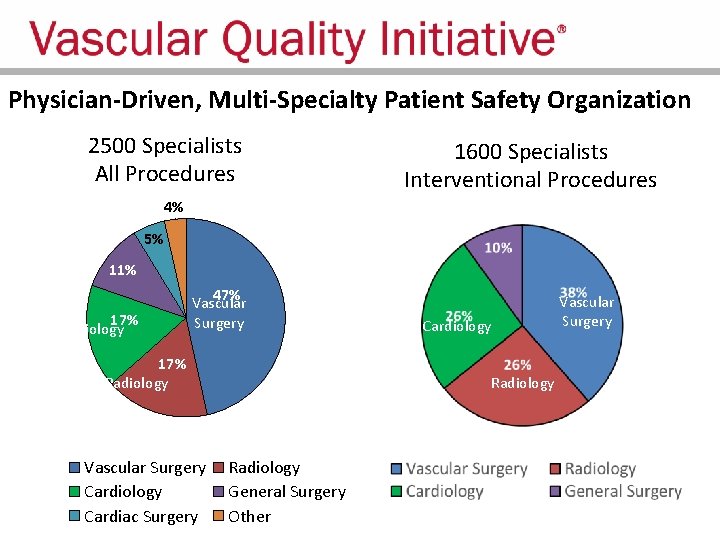
Physician-Driven, Multi-Specialty Patient Safety Organization 2500 Specialists All Procedures 1600 Specialists Interventional Procedures 4% 5% 11% 17% Cardiology 47% Vascular Surgery 17% Radiology Vascular Surgery Cardiology Cardiac Surgery Cardiology Radiology General Surgery Other Vascular Surgery

How to Grow and Sustain a Self-Funded PSO • Leverage “Big Data” from the national registry to power analyses of processes that lead to best outcomes • Promote physician practice change, ownership and connection to the PSO/registry by developing smaller, regional quality improvement groups

New England QI Group Model - 2002 • Semi-annual meetings of physicians, nurses, researchers and administrators • Analyze variation in process and outcomes among regional centers • Discuss potential causes for variation • Develop quality improvement projects in areas where substantial variation exists • Promote ownership, collaboration, and greater opportunity to translate data into practice change

Network of 17 Regional Quality Groups AK HI Semi-annual meetings, Review variation Regional quality improvement projects

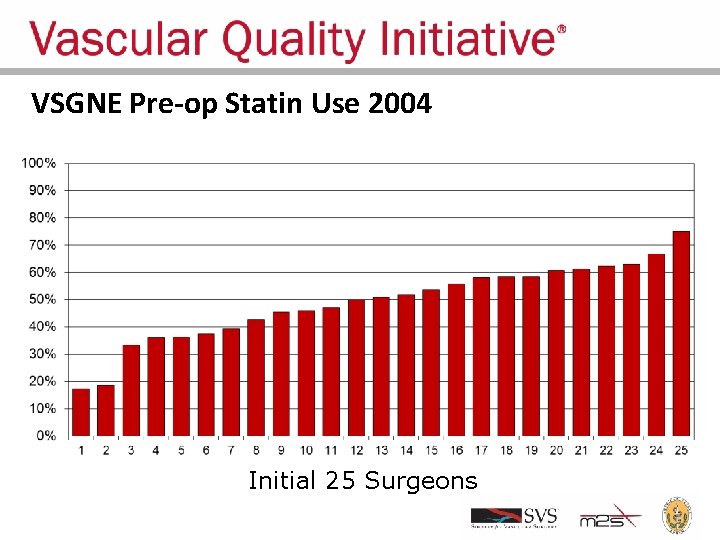
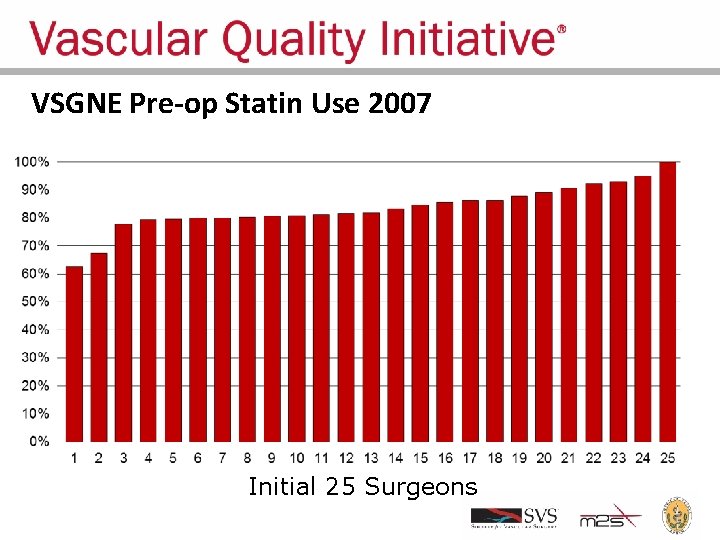
Regional Groups: Lessons Learned • Comparative feedback stimulates practice change – Physicians are naturally competitive – We all want to improve our results – We all want to have the best results • Most vascular patients should be on a statin pre-op – Record statin use – Feedback results to surgeons

VSGNE Pre-op Statin Use 2004 Initial 25 Surgeons

VSGNE Pre-op Statin Use 2007 Initial 25 Surgeons

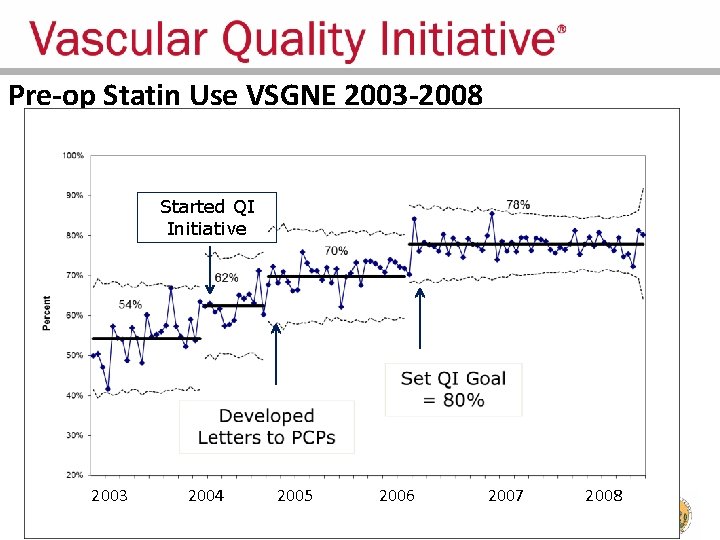
Pre-op Statin Use VSGNE 2003 -2008 Started QI Initiative 2003 2004 2005 2006 2007 2008

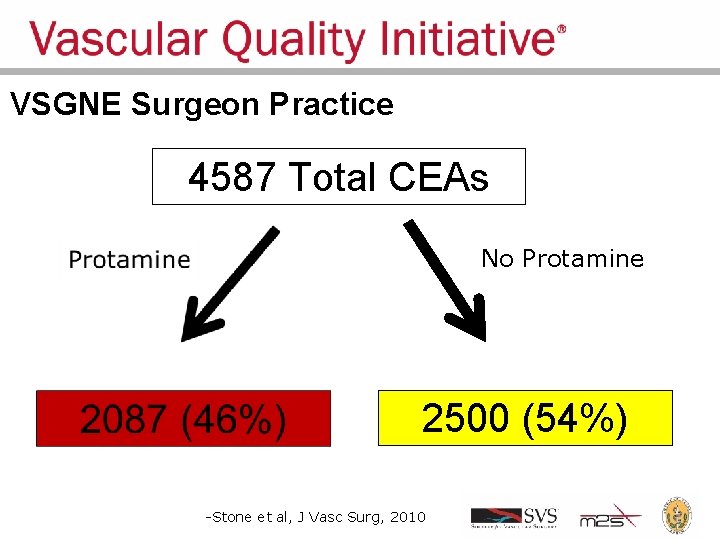
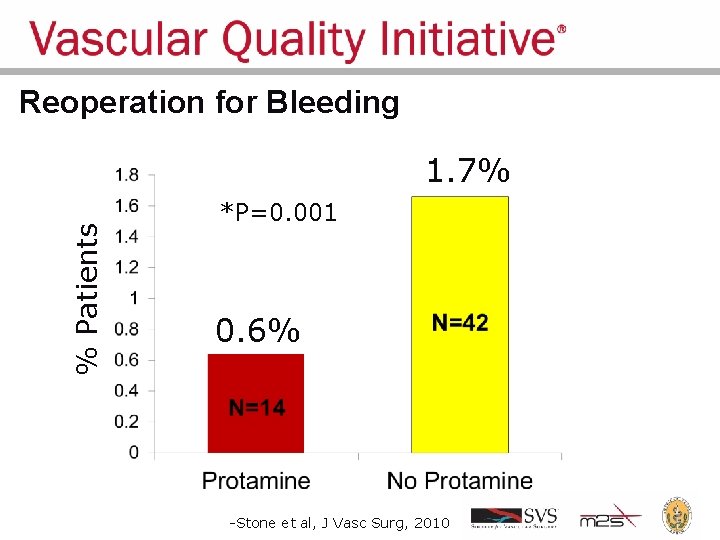
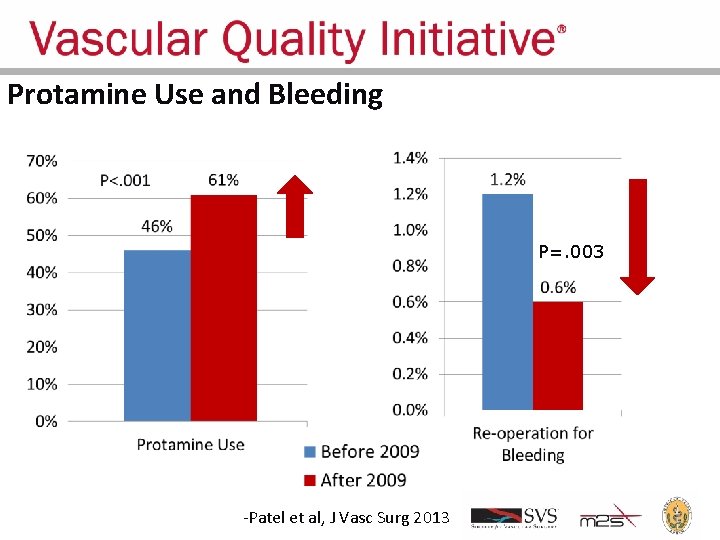
Regional Groups: Lessons Learned • Comparative feedback stimulates practice change • Large dataset can answer important clinical questions – Should I use protamine to reverse heparin during carotid endarterectomy? • Protamine reverses anticoagulant used during surgery, to promote clotting – Re-operation for bleeding: 1. 7% • Concern about causing too much clotting: stroke, MI – Low frequency events cannot be studies in small series and randomized trials are unrealistic

VSGNE Surgeon Practice 4587 Total CEAs No Protamine 2500 (54%) -Stone et al, J Vasc Surg, 2010

Reoperation for Bleeding % Patients 1. 7% *P=0. 001 0. 6% -Stone et al, J Vasc Surg, 2010

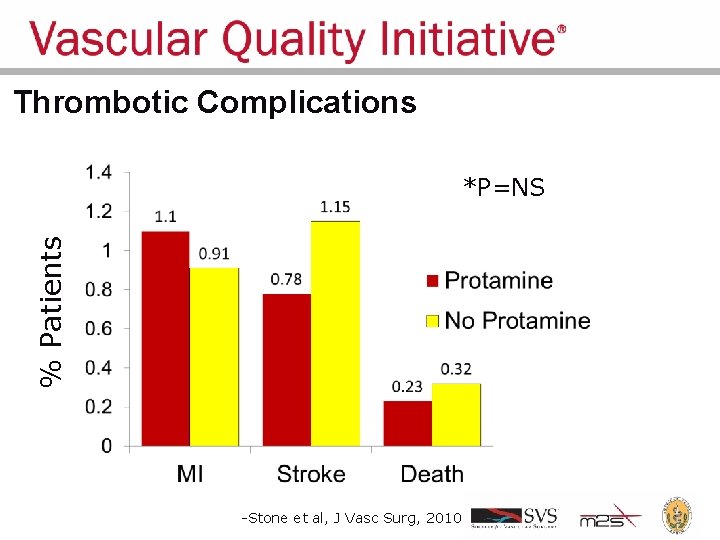
Thrombotic Complications % Patients *P=NS -Stone et al, J Vasc Surg, 2010

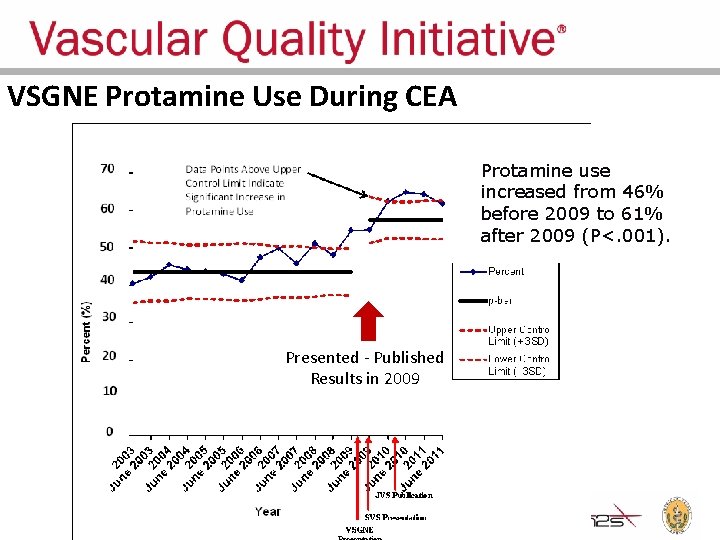
Regional Groups: Lessons Learned • Comparative feedback stimulates practice change • Large dataset can answer important clinical questions • Trusted analyses, reports can rapidly change practice – Physicians have ownership of regional group data – Protamine data were presented to regional group and published

VSGNE Protamine Use During CEA Protamine use increased from 46% before 2009 to 61% after 2009 (P<. 001). Presented - Published Results in 2009

Regional Groups: Lessons Learned • • Comparative feedback stimulates practice change Large dataset can answer important clinical questions Trusted analyses, reports can rapidly change practice Changed practice can improve outcomes

Protamine Use and Bleeding P=. 003 -Patel et al, J Vasc Surg 2013

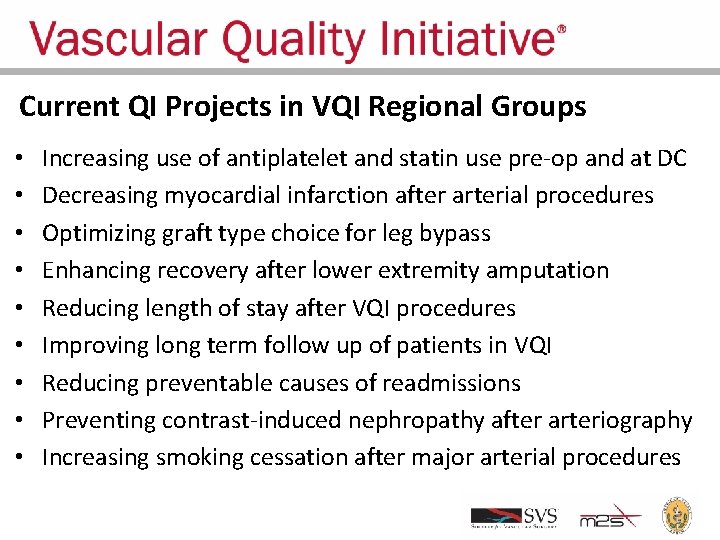
Current QI Projects in VQI Regional Groups • • • Increasing use of antiplatelet and statin use pre-op and at DC Decreasing myocardial infarction after arterial procedures Optimizing graft type choice for leg bypass Enhancing recovery after lower extremity amputation Reducing length of stay after VQI procedures Improving long term follow up of patients in VQI Reducing preventable causes of readmissions Preventing contrast-induced nephropathy after arteriography Increasing smoking cessation after major arterial procedures

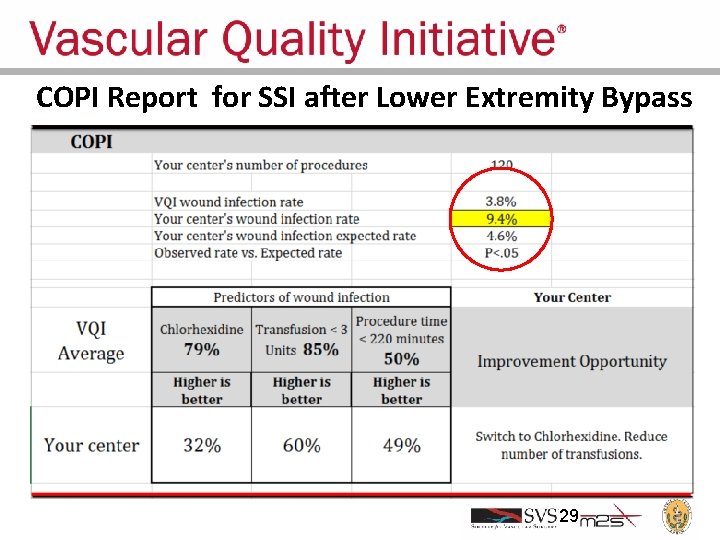
Improving Quality Across VQI Regions • COPI Reports – Center Opportunity Profile for Improvement • Analyze and report variation in outcome • Multivariable model to define causes of outcome • Individual report to each center: – How they compare with others for the outcome and each factor associated with the outcome – Provides a customized, actionable improvement plan for each center

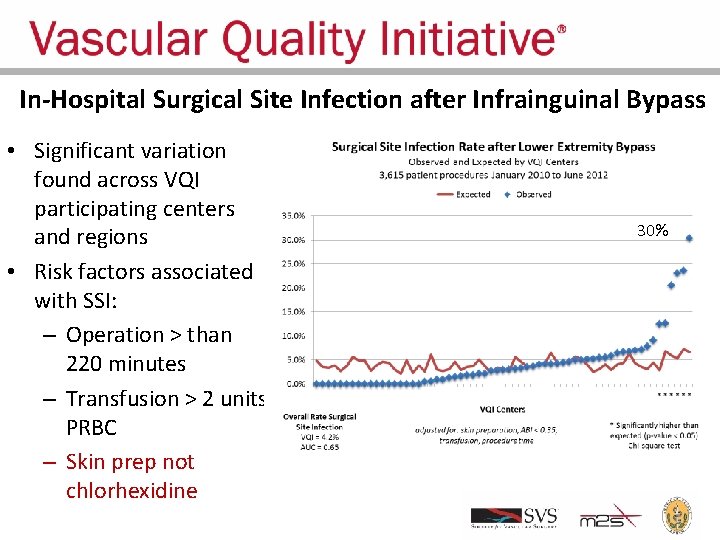
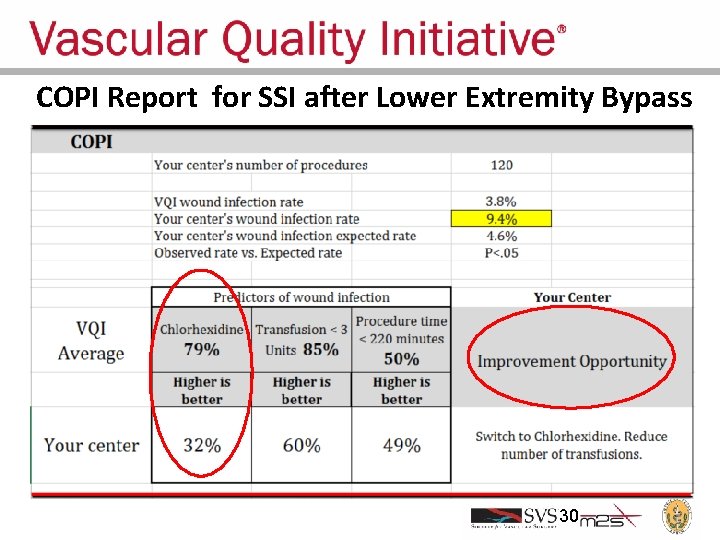
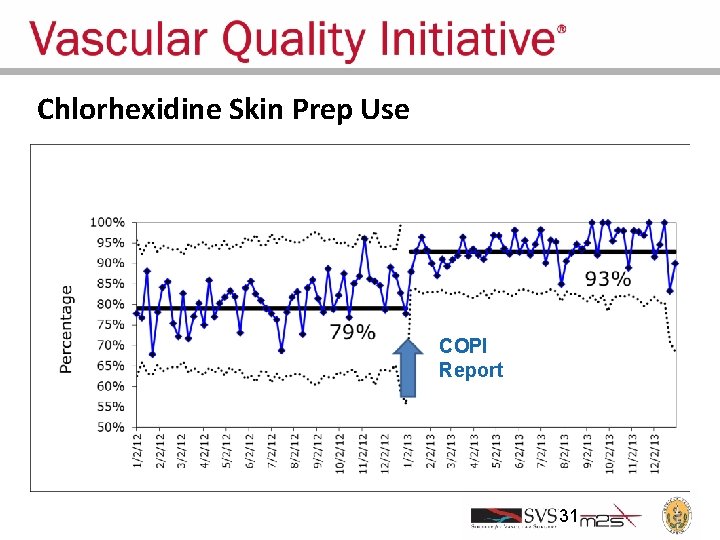
In-Hospital Surgical Site Infection after Infrainguinal Bypass • Significant variation found across VQI participating centers and regions • Risk factors associated with SSI: – Operation > than 220 minutes – Transfusion > 2 units PRBC – Skin prep not chlorhexidine 30%

COPI Report for SSI after Lower Extremity Bypass 29

COPI Report for SSI after Lower Extremity Bypass 30

Chlorhexidine Skin Prep Use COPI Report 31

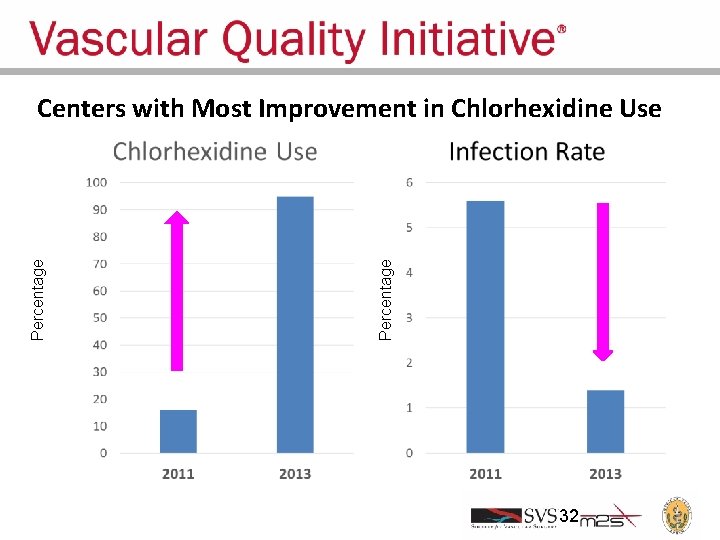
Percentage Centers with Most Improvement in Chlorhexidine Use 32

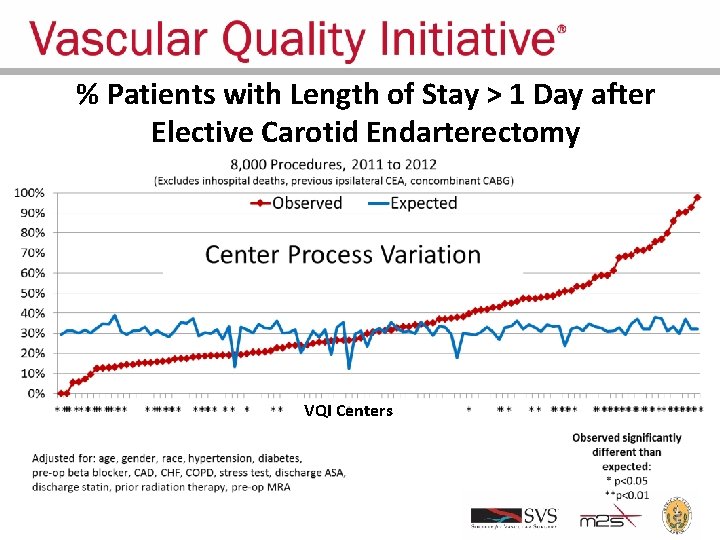
% Patients with Length of Stay > 1 Day after Elective Carotid Endarterectomy VQI Centers

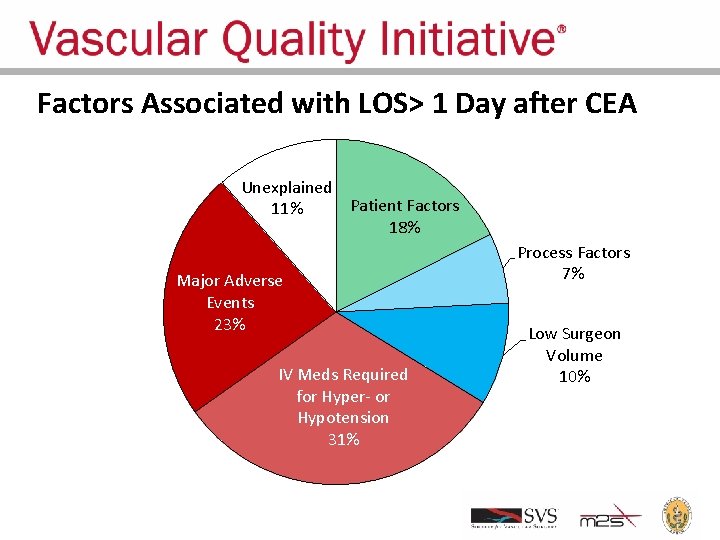
Factors Associated with LOS> 1 Day after CEA Unexplained Patient Factors 11% 18% Major Adverse Events 23% IV Meds Required for Hyper- or Hypotension 31% Process Factors 7% Low Surgeon Volume 10%

Value of VQI Participation • Does participation in VQI (receiving benchmark reports, attending regional meetings, etc. ) improve patient outcomes?

Late Survival after Major Arterial Procedures • 50, 000 Patients in VQI who underwent – Leg bypass / intervention, o. AAA / EVAR, CEA / CAS • Evaluated pre-operative and discharge medications: – Antiplatelet agent (ASA, PY 212 inhibitors) – Statins (HMG-Co. A reductase inhibitors) • Outcomes analyzed: – Effect on patient survival – Variation across centers – Impact of participation in VQI -De Martino et al, J Vasc Surg, 2015

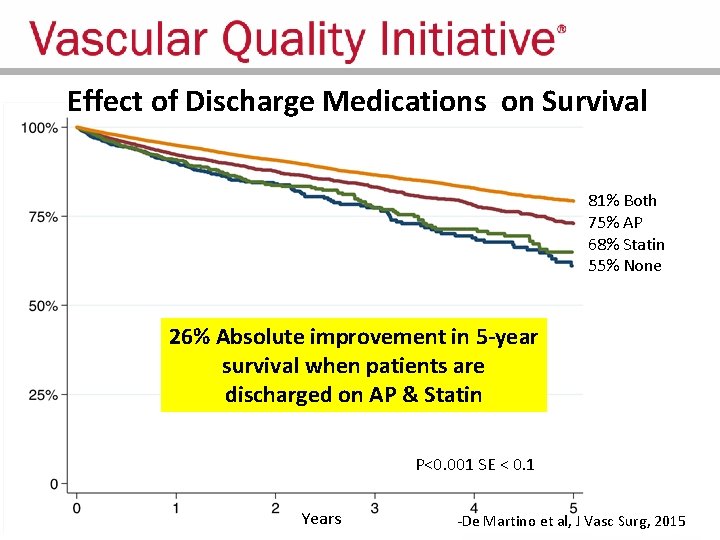
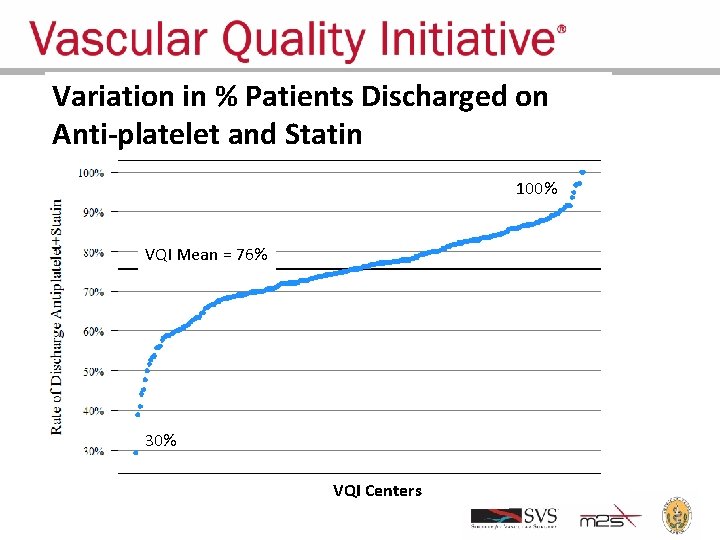
Effect of Discharge Medications on Survival 81% Both 75% AP 68% Statin 55% None 26% Absolute improvement in 5 -year survival when patients are discharged on AP & Statin P<0. 001 SE < 0. 1 Years -De Martino et al, J Vasc Surg, 2015

Variation in % Patients Discharged on Anti-platelet and Statin 100% VQI Mean = 76% 30% VQI Centers

Patients on Antiplatelet and Statin Pre-op and Discharge Based on Center Years Participation in VQI Number of Years Participating in VQI

New VQI Initiatives • Evaluating appropriateness of treatment

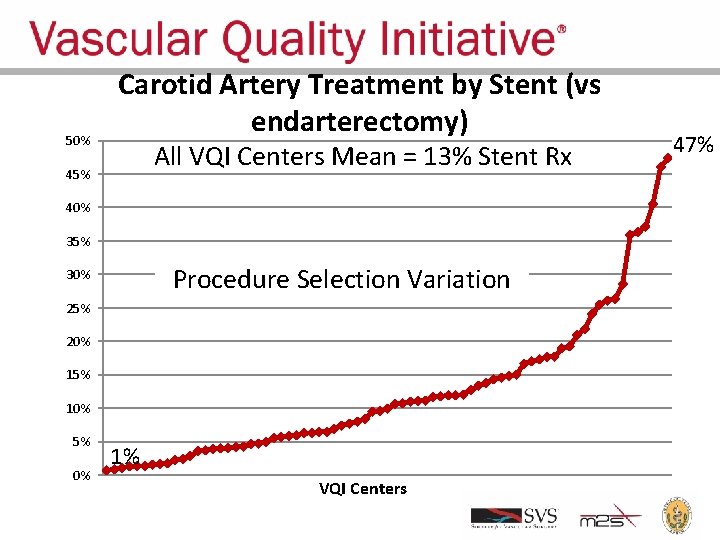
Appropriate Treatment • Appropriate treatment requires not only good early and late outcomes, but also: – Correct patient selection – Correct procedure selection • VQI provides an opportunity to analyze variation in patient and procedure selection – Feedback data to centers – Goal: regression toward the mean and reduced variation • Current data show large variation!

50% Carotid Artery Treatment by Stent (vs endarterectomy) All VQI Centers Mean = 13% Stent Rx 45% 40% 35% Procedure Selection Variation 30% 25% 20% 15% 10% 5% 0% 1% VQI Centers 47%

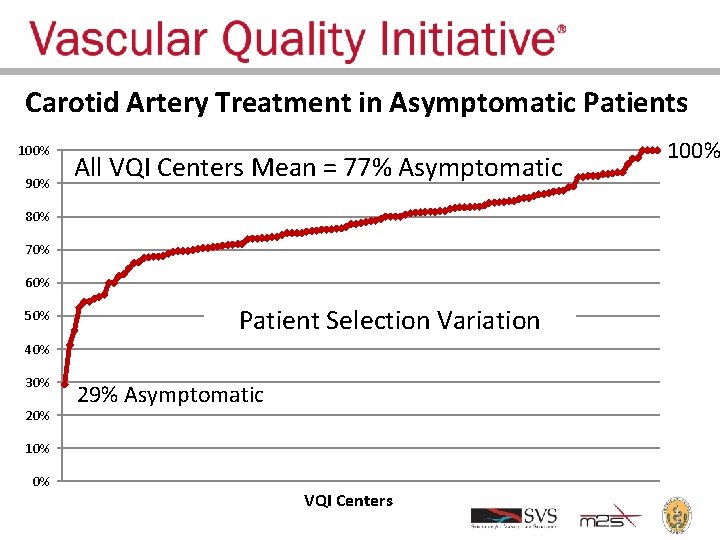
Carotid Artery Treatment in Asymptomatic Patients 100% 90% All VQI Centers Mean = 77% Asymptomatic 80% 70% 60% 50% Patient Selection Variation 40% 30% 29% Asymptomatic 10% 0% VQI Centers 100%

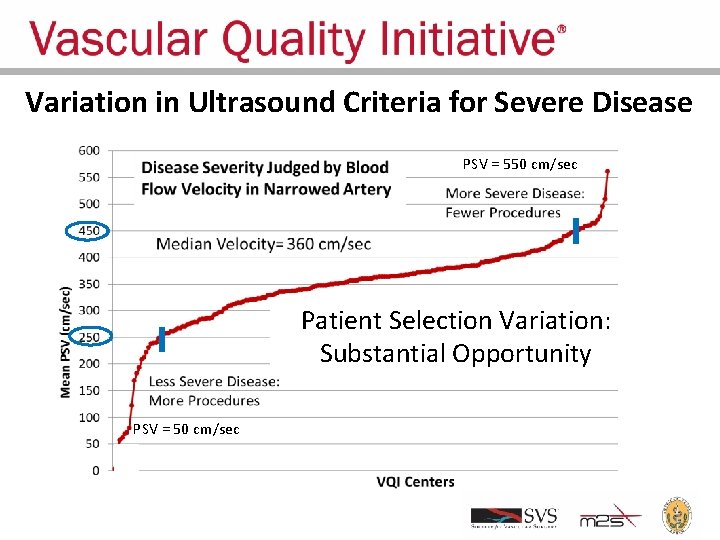
Variation in Ultrasound Criteria for Severe Disease PSV = 550 cm/sec Patient Selection Variation: Substantial Opportunity PSV = 50 cm/sec

New VQI Initiatives • Evaluating appropriateness of treatment • Evaluating new medical devices in real world practice

FDA, Device Manufacturer Collaboration • Many devices used to treat vascular disease (eg, stents) • Devices approved after limited testing in selected patients in centers of excellence • In practice, devices may be used outside indications for use and in less experienced centers • Data about device performance in real world practice can inform physicians, FDA and manufacturers, to optimize treatment benefits for patients • VQI is sharing non-identifiable data regarding new device outcomes with FDA and manufacturers

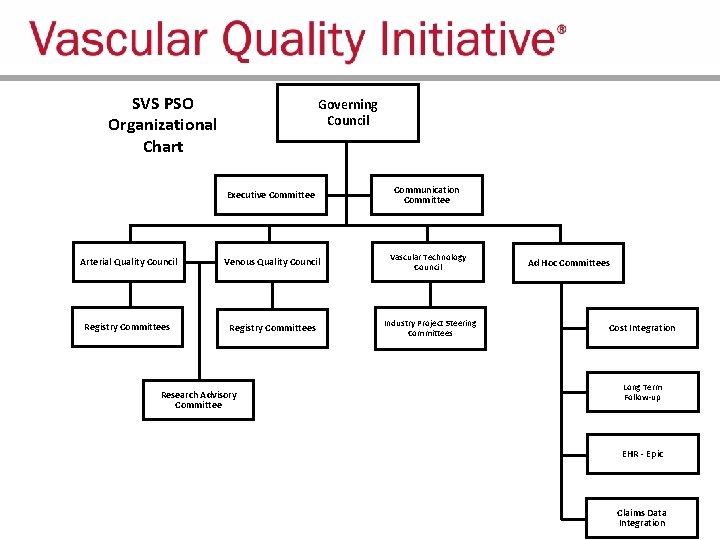
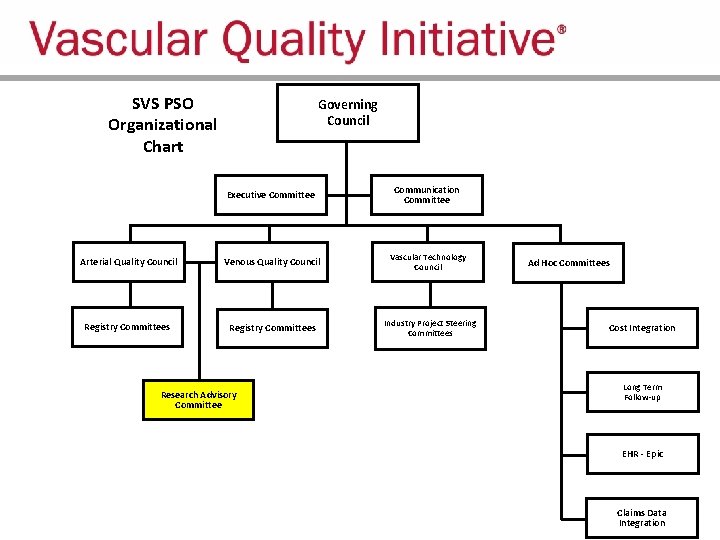
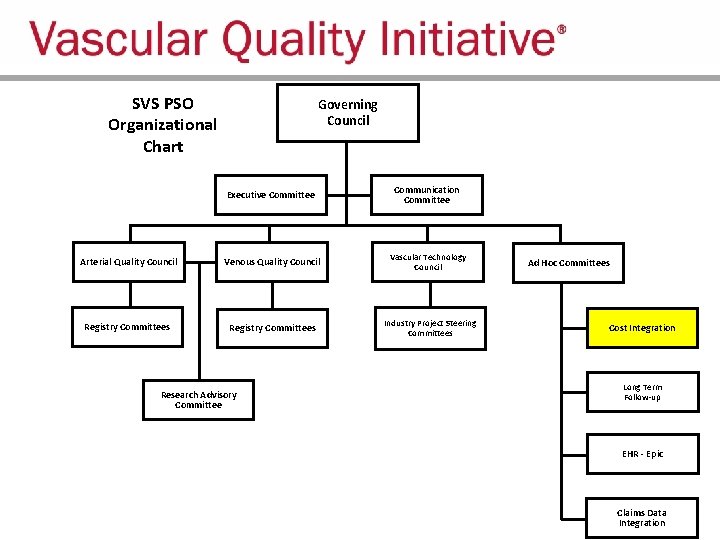
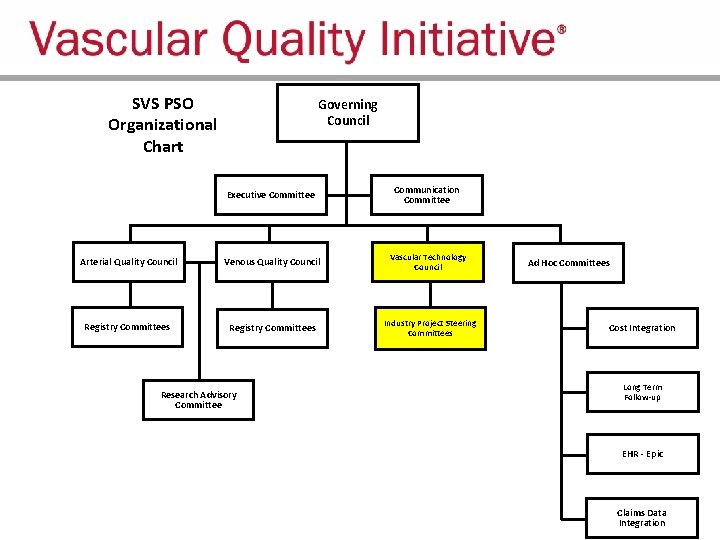
SVS PSO Organizational Structure

SVS PSO Organizational Chart Governing Council Executive Committee Communication Committee Arterial Quality Council Venous Quality Council Vascular Technology Council Registry Committees Industry Project Steering Committees Research Advisory Committee Ad Hoc Committees Cost Integration Long Term Follow-up EHR - Epic Claims Data Integration

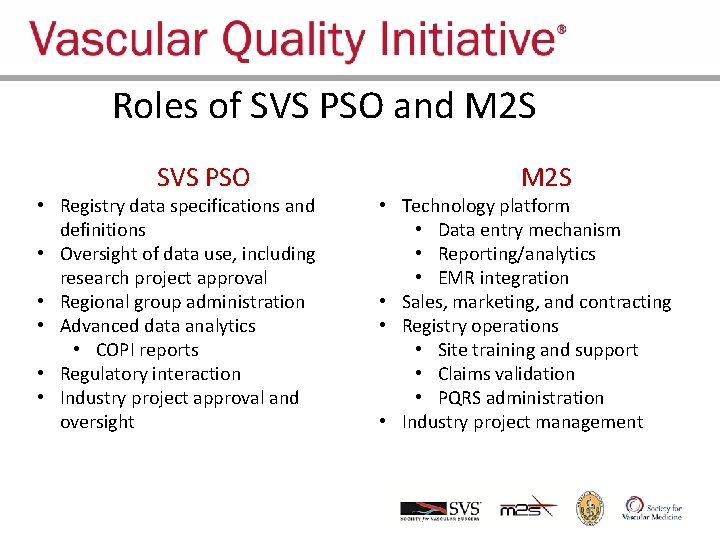
Roles of SVS PSO and M 2 SVQI SVS PSO • Registry data specifications and definitions • Oversight of data use, including research project approval • Regional group administration • Advanced data analytics • COPI reports • Regulatory interaction • Industry project approval and oversight M 2 S • Technology platform • Data entry mechanism • Reporting/analytics • EMR integration • Sales, marketing, and contracting • Registry operations • Site training and support • Claims validation • PQRS administration • Industry project management

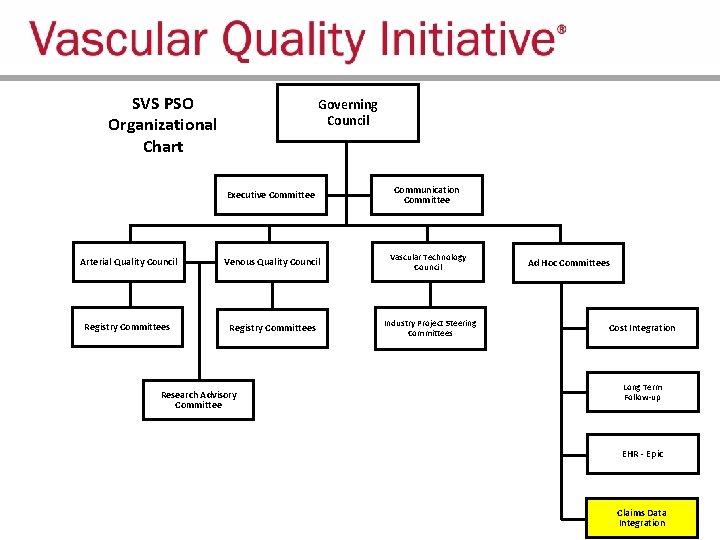
SVS PSO Organizational Chart Governing Council Executive Committee Communication Committee Arterial Quality Council Venous Quality Council Vascular Technology Council Registry Committees Industry Project Steering Committees Research Advisory Committee Ad Hoc Committees Cost Integration Long Term Follow-up EHR - Epic Claims Data Integration

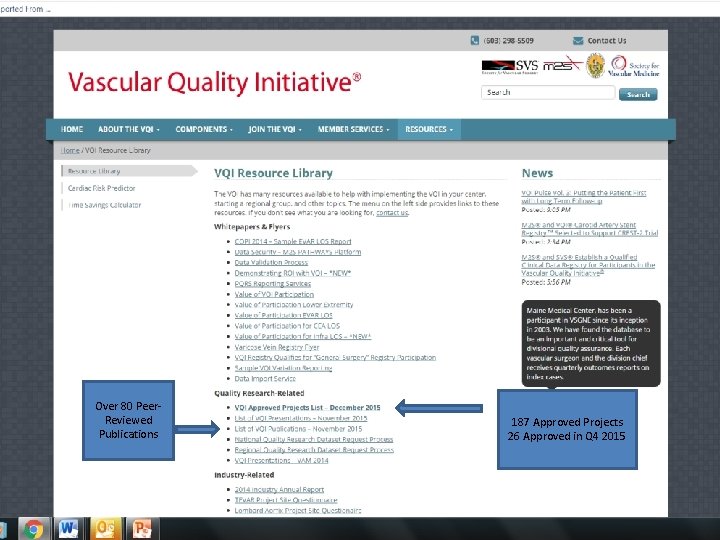
Over 80 Peer. Reviewed Publications 187 Approved Projects 26 Approved in Q 4 2015

SVS PSO Organizational Chart Governing Council Executive Committee Communication Committee Arterial Quality Council Venous Quality Council Vascular Technology Council Registry Committees Industry Project Steering Committees Research Advisory Committee Ad Hoc Committees Cost Integration Long Term Follow-up EHR - Epic Claims Data Integration

Matching Medicare Claims Data with VQI Registry • Goal: determine late outcomes after initial vascular treatment using Medicare claims related to subsequent treatment • Dartmouth Institute for Health Policy and Clinical Practice • Match claims data identifiers with VQI patient identifiers: – CEA, CAS, EVAR, o. AAA, TEVAR, PVI, leg bypass datasets – Match rates > 90% for procedures done 2003 -2013, age > 65 • Social Security Death Index also matched in patients of all ages • Data available for VQI based research and industry projects

Matching Medicare Claims Data with VQI Registry • Major outcomes and time to event after index procedure: • Carotid (CEA and CAS) – Re-admission, death, stroke, re-intervention (CEA or CAS), imaging • Aorta (o. AAA, EVAR, TEVAR) – Re-admission, death, re-intervention (open or EVAR), abdominal surgery for complication after o. AAA • Leg (Infra, Supra, PVI) – Re-admission, death, re-intervention (open or EVAR), imaging, amputation (major, minor)

SVS PSO Organizational Chart Governing Council Executive Committee Communication Committee Arterial Quality Council Venous Quality Council Vascular Technology Council Registry Committees Industry Project Steering Committees Research Advisory Committee Ad Hoc Committees Cost Integration Long Term Follow-up EHR - Epic Claims Data Integration

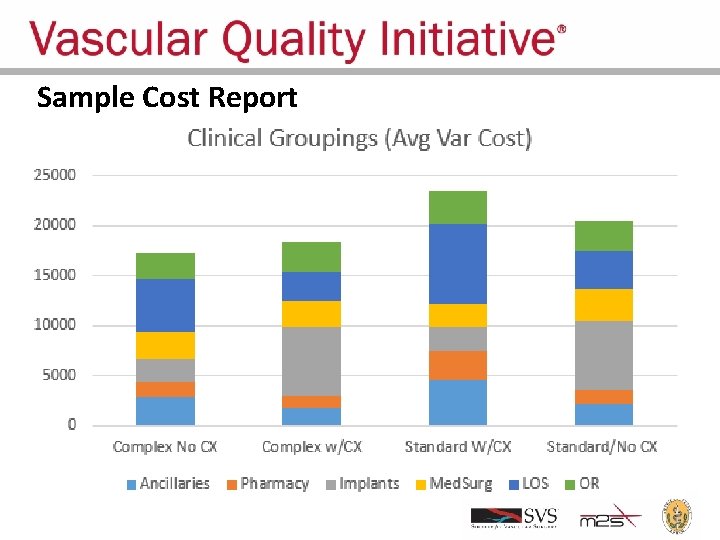
Estimating Cost for Procedures Performed in VQI • Goal: Provide centers with anonymous benchmark comparison of treatment costs when adjusted for disease severity and procedure details already collected in VQI • Method: Partnered with Med. Assets, a healthcare performance improvement company that focuses on finance and operations • Pilot Project: – 17 VQI hospitals submitted UB-04 claims for 2014 EVARs – Using VQI clinical data, cases grouped as standard or complex, and without post-treatment complications – Med. Assets calculated costs from charge data using usual methods • Currently preparing reports of total and component cost • Plan to offer for all procedures for all centers

Sample Cost Report

SVS PSO Organizational Chart Governing Council Executive Committee Communication Committee Arterial Quality Council Venous Quality Council Vascular Technology Council Registry Committees Industry Project Steering Committees Research Advisory Committee Ad Hoc Committees Cost Integration Long Term Follow-up EHR - Epic Claims Data Integration

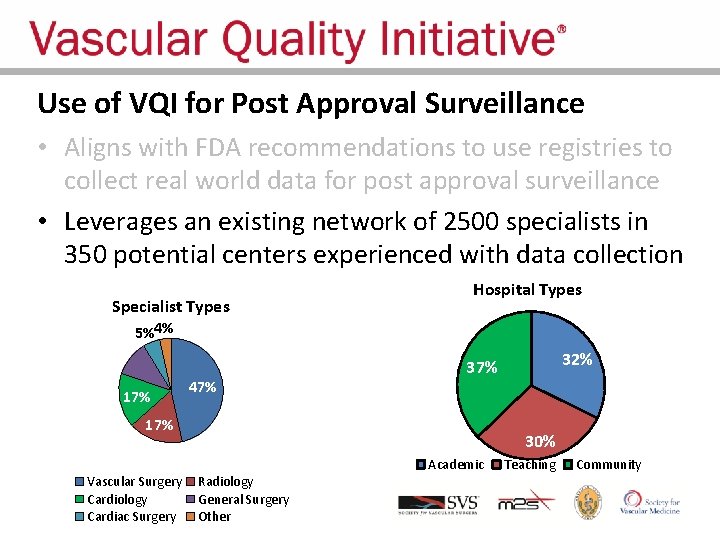
Use of VQI for Post Approval Surveillance • Aligns with FDA recommendations to use registries to collect real world data for post approval surveillance • “FDA believes that device registries should serve as the foundation of our National Medical Device Postmarket Surveillance System. ”

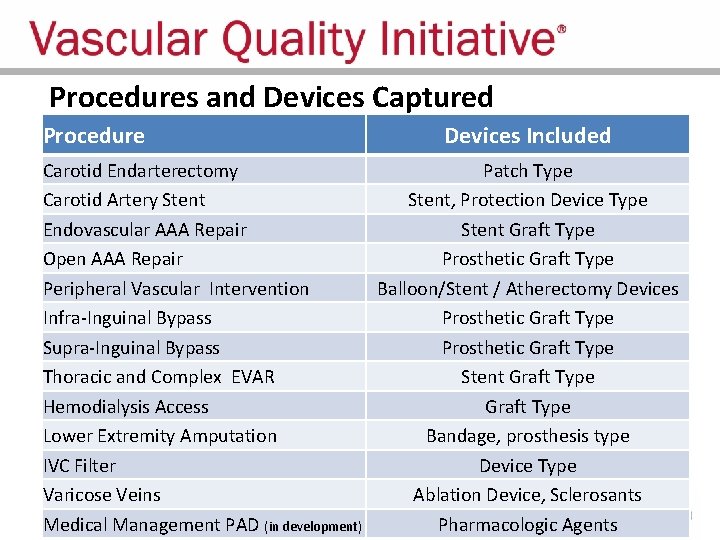
Procedures and Devices Captured Procedure Devices Included Carotid Endarterectomy Patch Type Carotid Artery Stent, Protection Device Type Endovascular AAA Repair Stent Graft Type Open AAA Repair Prosthetic Graft Type Peripheral Vascular Intervention Balloon/Stent / Atherectomy Devices Infra-Inguinal Bypass Prosthetic Graft Type Supra-Inguinal Bypass Prosthetic Graft Type Thoracic and Complex EVAR Stent Graft Type Hemodialysis Access Graft Type Lower Extremity Amputation Bandage, prosthesis type IVC Filter Device Type Varicose Veins Ablation Device, Sclerosants Medical Management PAD (in development) Pharmacologic Agents

Use of VQI for Post Approval Surveillance • Aligns with FDA recommendations to use registries to collect real world data for post approval surveillance • Leverages an existing network of 2500 specialists in 350 potential centers experienced with data collection Specialist Types 11% Hospital Types 5%4% 17% 47% 17% Vascular Surgery Cardiology Cardiac Surgery 32% 37% 30% Radiology General Surgery Other Academic Teaching Community

Use of VQI for Post Approval Surveillance • Aligns with FDA recommendations to use registries to collect real world data for post approval surveillance. • Leverages an existing network of 350 potential centers and 2600 specialists experienced with data collection • Uses existing VQI data forms supplemented as needed with additional variables, follow-up time points – Customized content for participating sites – New sites can join VQI for project if not already member

Use of VQI for Post Approval Surveillance • Aligns with FDA recommendations to use registries to collect real world data for post approval surveillance • Leverages an existing network of 350 potential centers and 2600 specialists experienced with data collection • Uses existing VQI data forms supplemented as needed with additional variables, follow-up time points • Informed consent and IRB approval NOT required – Standard of care practice and PSO Quality Project – Allows all patients treated in center to be captured

Use of VQI for Post Approval Surveillance • Aligns with FDA recommendations to use registries to collect real world data for post approval surveillance • Leverages an existing network of 350 potential centers and 2600 specialists experienced with data collection • Uses existing VQI data forms supplemented as needed with additional variables, follow-up time points • Informed consent and IRB approval NOT required • SVS PSO contracts with sites, facilitates recruitment – Uniform contract, no separate site negotiations

Use of VQI for Post Approval Surveillance • Aligns with FDA recommendations to use registries to collect real world data for post approval surveillance • Leverages an existing network of 350 potential centers and 2600 specialists experienced with data collection • Uses existing VQI data forms supplemented as needed with additional variables, follow-up time points • Informed consent and IRB approval NOT required • SVS PSO contracts with sites, facilitates recruitment • Result = Rapid, Efficient, Flexible, Cost-Effective

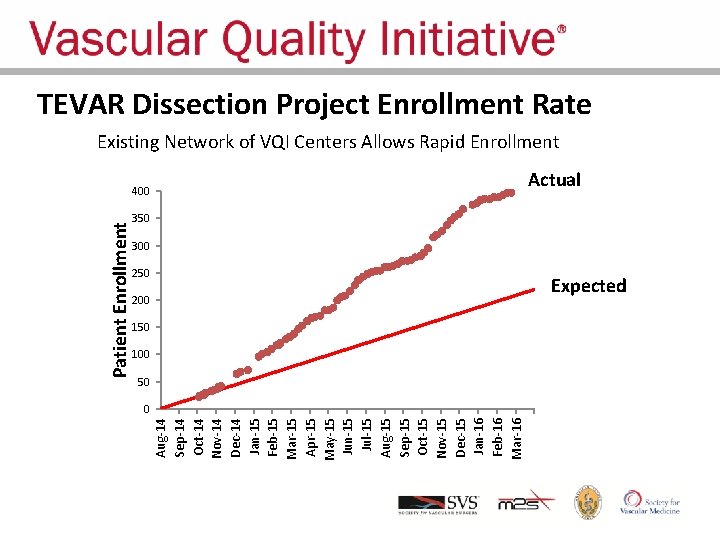
Aug-14 Sep-14 Oct-14 Nov-14 Dec-14 Jan-15 Feb-15 Mar-15 Apr-15 May-15 Jun-15 Jul-15 Aug-15 Sep-15 Oct-15 Nov-15 Dec-15 Jan-16 Feb-16 Mar-16 Patient Enrollment TEVAR Dissection Project Enrollment Rate Existing Network of VQI Centers Allows Rapid Enrollment 400 Actual 350 300 250 200 Expected 150 100 50 0

Project Steering Committee • Scientific Steering Committee is appointed by SVS PSO with input from sponsor to insure experienced users • Steering Committee reviews data and interacts with sites to adjudicate any data questions during analysis • Steering Committee takes responsibility for scientific presentation and publication of final data analysis in consultation with sponsor • Coordinated by specific Project Manager at M 2 S

Protocol Development, Site Selection • Collaboration between SVS PSO Steering Committee, FDA and the sponsor helps design a pragmatic surveillance protocol – Data collection requirements that fit real world registries – Follow-up time points appropriate for standard of care • Steering Committee selects optimal sites for project – Physicians who have been trained to use the new device – Sites which meet resource requirements for data entry – Sites with known accurate and timely VQI data collection • M 2 S Project Team monitors site performance, provides feedback

Data Ownership • SVS PSO provides sponsor with copy of “line by line” data – De-identified for patient, provider, hospital – Dates converted to intervals since procedure, age, etc. • Sponsor may use data for analysis, regulatory requirements and marketing – Marketing content must be approved by SVS PSO when source of data is cited to insure accurate interpretation • Steering Committee produces scientific presentations, publications, in consultation with sponsor

Additional Industry Uses for VQI Data: Supplementing Historical Data to Reduce Project Length • Substantial data exists in VQI but might be missing some custom variables needed for a specific device project • It is possible for sites to retrieve and add custom data elements to previously entered data forms, including additional follow-up • VQI identifies relevant procedures, invites sites to supplement data for required number of procedures • This allows projects to rapidly accrue procedures and shorten time required for follow-up and study completion • One recent project completed, developed efficient on-line mechanism for sites to identify and enter needed data

Additional Industry Uses for VQI Data: Using Registry Data to Expand Device Indications • Many devices have been applied to arteries or patients not tested in initial trials, and thus used “off-label” – Biliary stents in peripheral arteries, veins – EVAR devices for short, angulated aortic neck • Individual physicians make decisions based on limited data • FDA recognizes need for a more efficient method to expand indications potentially based on comprehensive registry data • Initial project underway to establish additional indication in PVI treatment of SFA disease, without IDE requirement

Additional Industry Uses for VQI Data: Use of VQI for Premarket Clinical Trials • Must be conducted according to 21 CFR 812 (IRB approval, informed consent, investigational site monitoring, investigator and sponsor responsibilities, adverse event reporting and submission of periodic reports to FDA and IRBs) • Benefit to leverage existing VQI network and avoid double data entry by sites into both VQI registry and industry CRF • Uses existing VQI data forms supplemented as needed with additional variables, follow-up time points • Opportunity to reduce study cost and time simply by using VQI for electronic data capture

Additional Industry Uses for VQI Data: FDA Medical Device Reporting • Current MDR system is costly, does not capture all device events, and provides no denominator to calculate event rates • Potential exists to use registry to identify potential adverse device-related events: – Capture “potential” serious device-related events by: • Death, re-intervention, excess imaging studies after index procedure • Use both registry and claims data – Fund centers to provide additional information about events • Drill down to establish cause of event and any device contribution • Current discussions with FDA about potential pilot project

Conclusions The PSO structure facilitates growth of QI initiative Regional groups promote physician ownership, trust Comparative data stimulates practice change Big Data provides new information COPI reports provide actionable, site specific opportunities for change that improves outcomes • Variation in appropriateness can be measured and will hopefully lead to better patient, procedure selection • PSO data can serve multiple stakeholders for optimal efficiency • • •

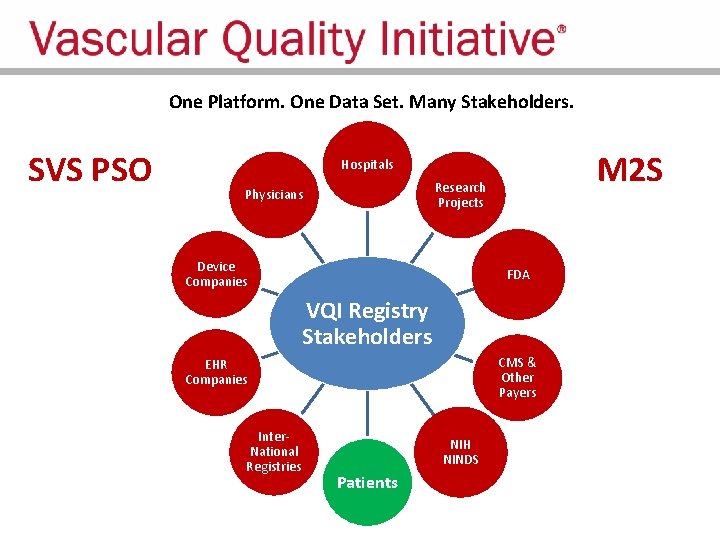
One Platform. One Data Set. Many Stakeholders. SVS PSO M 2 S Hospitals Research Projects Physicians Device Companies FDA VQI Registry Stakeholders CMS & Other Payers EHR Companies Inter. National Registries NIH NINDS Patients