The use of Hydrogen Peroxide for Colouring and












- Slides: 13

The use of Hydrogen Peroxide for Colouring and Bleaching

Hydrogen Peroxide. • Mixed with both permanent and quasi colours and bleaches. • It is known as an oxidising agent, which means once it is mixed with hair colours or bleaches, it releases oxygen. • Chemical Formula for hydrogen peroxide is H 202, during mixing it breaks down from H 202 to form H 20 + 0 (Water and Oxygen).

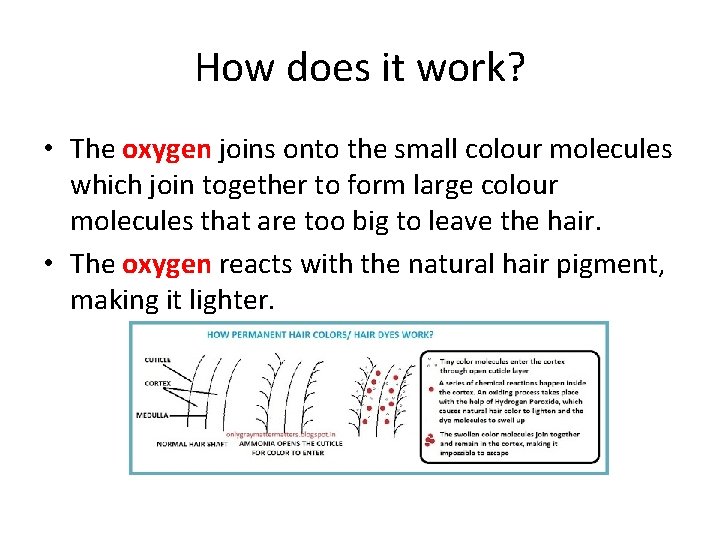
How does it work? • The oxygen joins onto the small colour molecules which join together to form large colour molecules that are too big to leave the hair. • The oxygen reacts with the natural hair pigment, making it lighter.

Types of Peroxide • Cream Peroxide: White creamy consistency, contains thickeners and acid stabilisers , making the hydrogen peroxide easier to control.

Types of Peroxide • Liquid Peroxide: Clear water like substance, that contains acid stabilisers, which when mixed with alkali (tint or bleach), speed up the release of the oxygen.

Hydrogen Peroxide Strengths • The strength of Hydrogen peroxide can be expressed in two ways: • Percentage Strength (%): This tells you how much pure hydrogen peroxide is in the solution • Example: In every 100 ml of 3% hydrogen peroxide 3% (3 ml) will be pure hydrogen peroxide and 97% will be water

Hydrogen Peroxide Strengths • Volume Strength (Vol): Tells you how much oxygen is released from 1 ml of hydrogen peroxide. • Examples: • 1 ml of 10 vol. gives 10 ml of oxygen. • 1 ml of 20 vol. gives 20 ml of oxygen.

Hydrogen Peroxide Strengths The stronger the solution: • The more pure hydrogen peroxide it contains. • The more oxygen can be released in the hair shaft.

Hydrogen Peroxide Strengths • • • 3% = 10 volume 6% = 20 volume 9% = 30 volume 12% = 40 volume 18% = 60 volume (the use of this is not prohibited in Europe and should not be used on the hair)

Hydrogen Peroxide Strengths

Diluting Hydrogen Peroxide • Hydrogen Peroxide can be diluted to make a weaker strength solution. For this process distilled water must be used as tap water may contain additives which may affect the colour result.

Storing Hydrogen Peroxide • If hydrogen peroxide is not stored properly it can lose its strength and will not work properly. • Keep containers tightly closed and replace lids as soon as possible after use. • Measure out what you need, do not pour any back into the bottle as it can pick up dust. • Store in a cool dark place.

Following Manufacturers Instructions • Always follow Manufacturers Instructions: • Not all mixing instructions will be the same. • Manufacturers may make changes to colouring products to update and improve them.