The Structure of Matter Compounds and Molecules What


- Slides: 13

The Structure of Matter Compounds and Molecules

What are Compounds? • Compounds are 2 or more elements chemically bonded together

Mixtures • Physical Blend of two or more pure substances • Elements and compounds

Examples of Molecular Substances • Many examples

Compounds • Chemical Bonds are forces that hold compounds together • A compound always has the same chemical formula • H 2 O • Na. Cl • KNO 3 • CO 2

Reading Chemical Formulas • Chemical formulas tell us a lot. • The subscripts tell us how many atoms or ions are in the compound • A compound always has the same chemical formula • H 2 O • Na. Cl • KNO 3 • CO 2 • HCl

Chemical Structure-shows how compounds atoms or ions are connected Positions of Atoms 1) Bond Length- is the distance between nucleus 2) Bond Angle- is the orientation in space

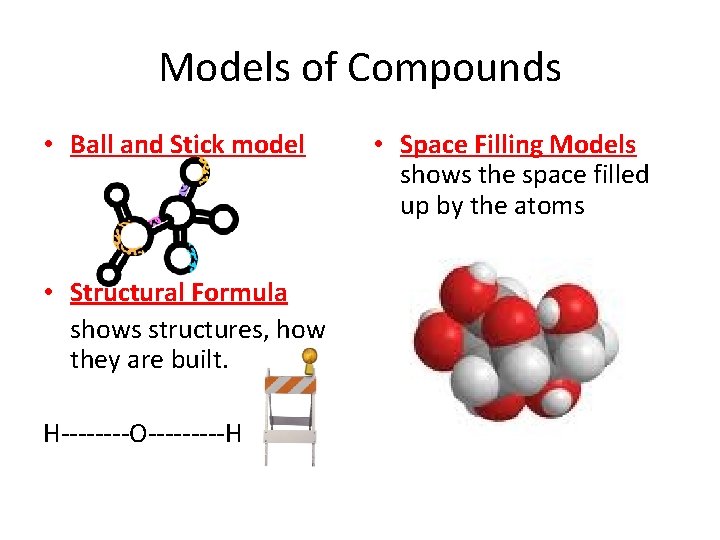
Models of Compounds • Ball and Stick model • Structural Formula shows structures, how they are built. H----O-----H • Space Filling Models shows the space filled up by the atoms

How does Structure affect Properties? • • States (solids, liquids, gases) Melting points Boiling points Freezing Points

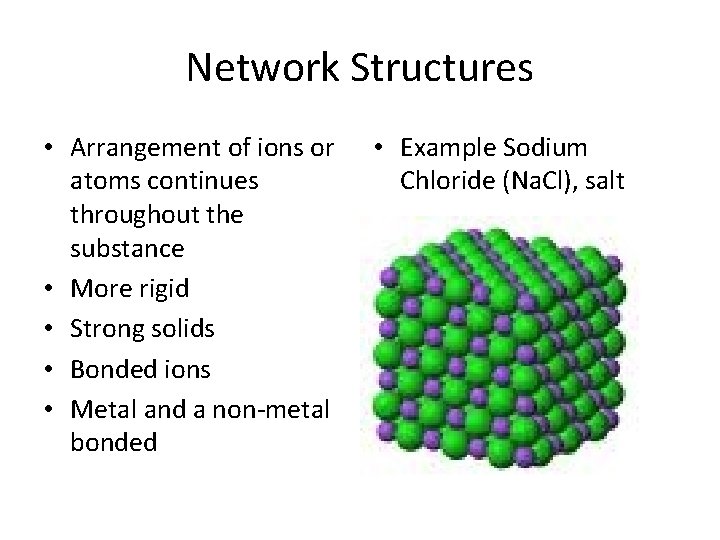
Network Structures • Arrangement of ions or atoms continues throughout the substance • More rigid • Strong solids • Bonded ions • Metal and a non-metal bonded • Example Sodium Chloride (Na. Cl), salt

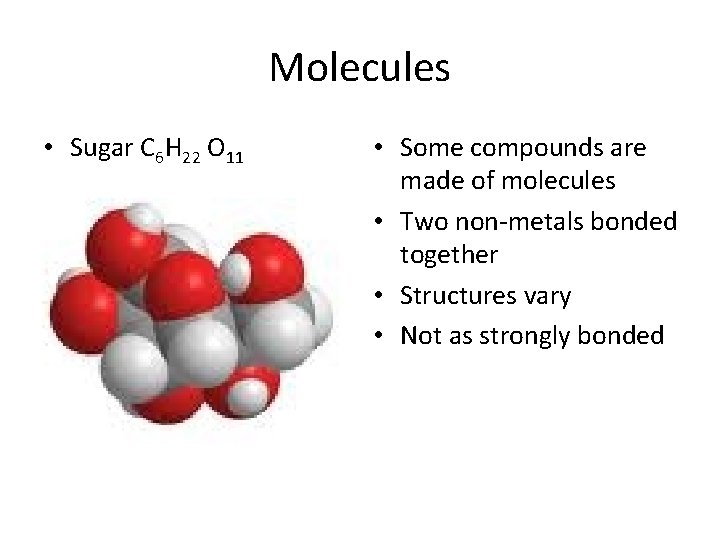
Molecules • Sugar C 6 H 22 O 11 • Some compounds are made of molecules • Two non-metals bonded together • Structures vary • Not as strongly bonded

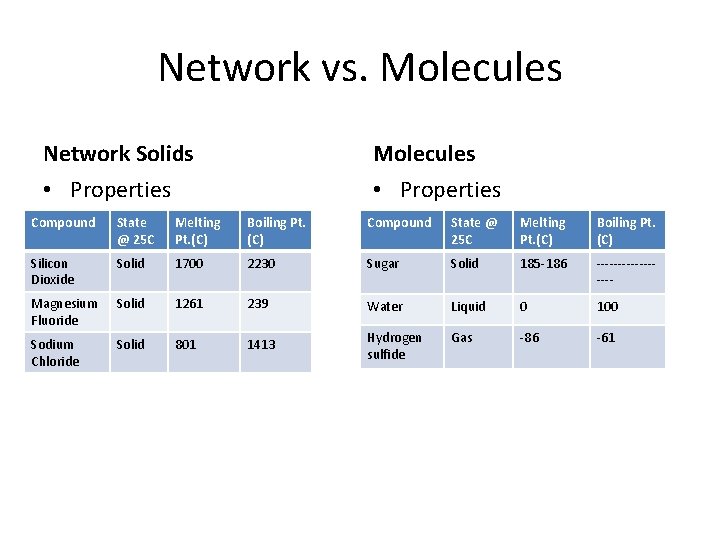
Network vs. Molecules Network Solids Molecules • Properties Compound State @ 25 C Melting Pt. (C) Boiling Pt. (C) Silicon Dioxide Solid 1700 2230 Sugar Solid 185 -186 --------- Magnesium Fluoride Solid 1261 239 Water Liquid 0 100 Sodium Chloride Solid 801 1413 Hydrogen sulfide Gas -86 -61

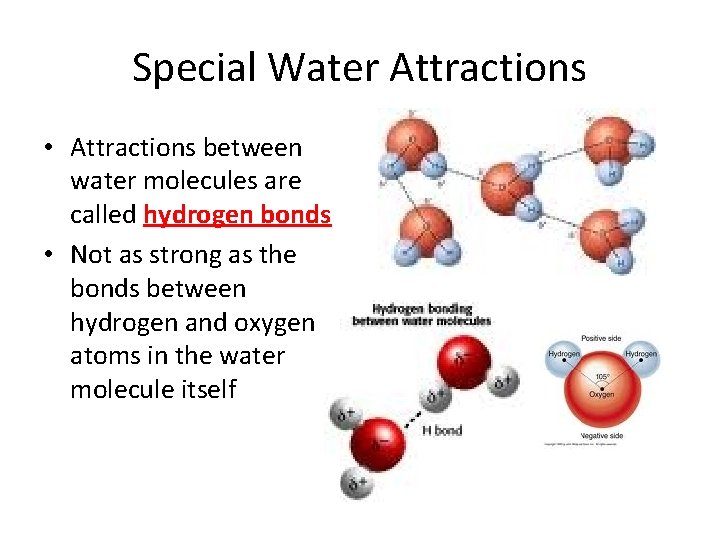
Special Water Attractions • Attractions between water molecules are called hydrogen bonds • Not as strong as the bonds between hydrogen and oxygen atoms in the water molecule itself