The Structure of Iron and Steel Pure Iron








- Slides: 14

The Structure of Iron and Steel

Pure Iron (Ferrite) Has approx. 0. 002% carbon Used since about 2000 BC Is stronger than most other pure metals. Made into weapons, armour, cooking pots and vessels • It is soft and ductile and imparts these properties to the steel. • In the pure state it is a very soft grey metal. • Of no commercial use. • •

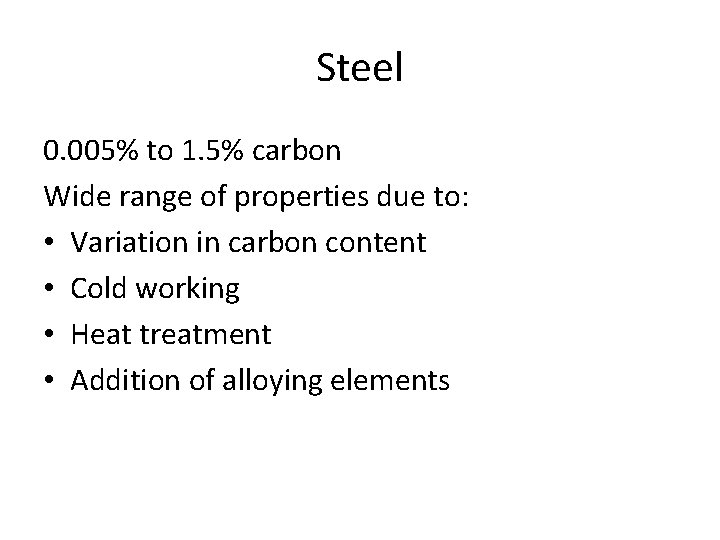
Steel 0. 005% to 1. 5% carbon Wide range of properties due to: • Variation in carbon content • Cold working • Heat treatment • Addition of alloying elements

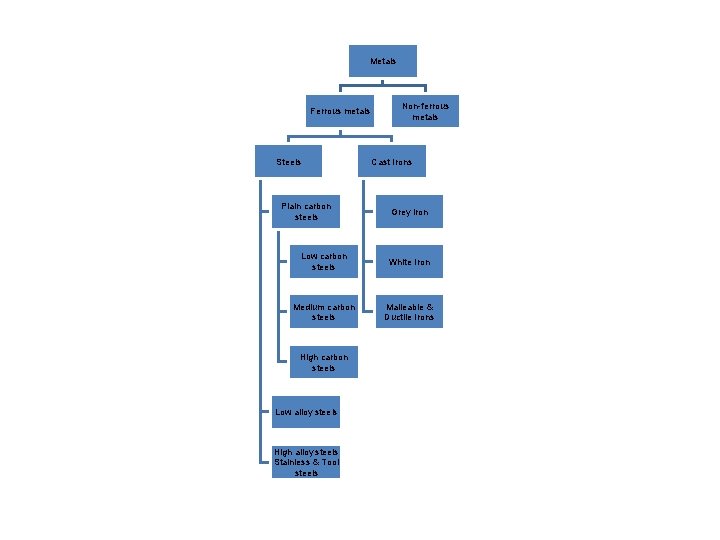
Metals Ferrous metals Steels Plain carbon steels Non-ferrous metals Cast Irons Grey Iron Low carbon steels White Iron Medium carbon steels Malleable & Ductile Irons High carbon steels Low alloy steels High alloy steels Stainless & Tool steels

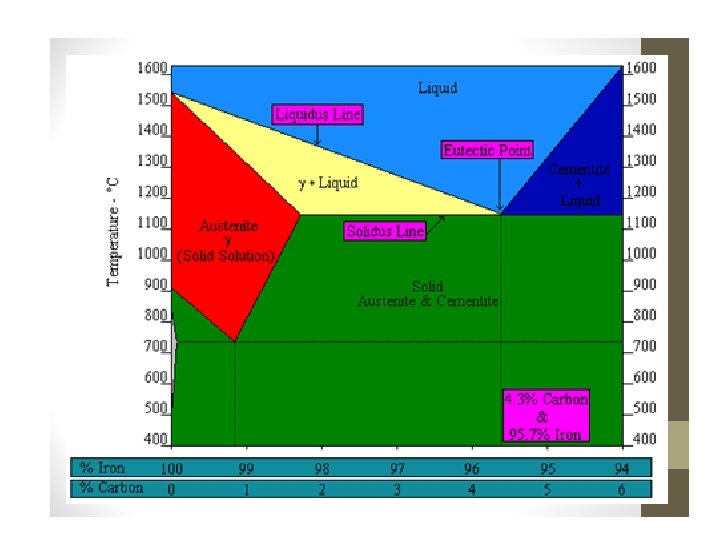
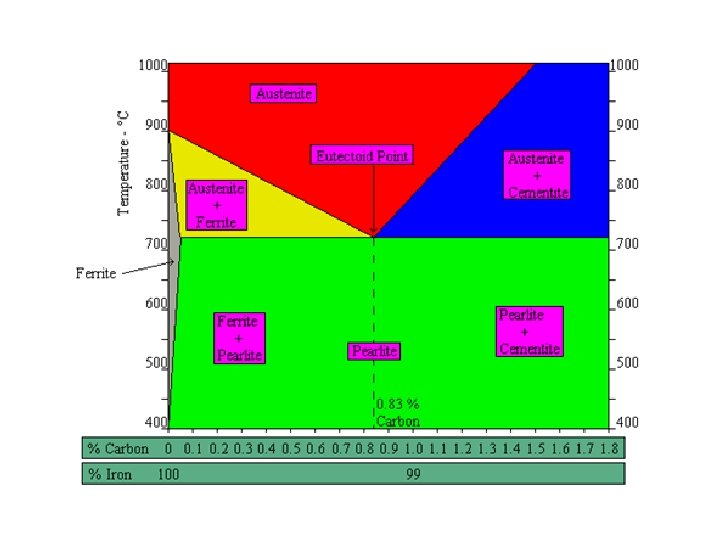
What Happens to Steel During Heating? • Heat treating of steel involves phase transformations. the phases are ferrite, austenite, cementite and pearlite. • A phase diagram simply helps us predict what will happen to the internal structure of the steel.

Microstructure of Steel Five main constituents: • Ferrite • Austenite • Cementite • Pearlite • Martensite



• At room temperature iron is ferrite which has a body-centred cubic (bcc) crystal structure. • Ferrite is a fairly soft material that can dissolve only a very small amount of carbon – (0. 021% at 910 °C and only 0. 008% at room temperature). • Ferrite is the phase that exists below the upper critical temperature of a steel with less than 0. 80% carbon.

• The formation of austenite begins when steel is heated above its lower critical temperature. • Austenite has a face-centred cubic (fcc) crystal structure and can contain up to 2% carbon at 1154°C. • Carbon strengthens steel and gives it the ability to be hardened by heat treatment.

• As carbon-rich austenite cools, the mixture attempts to revert back to the ferrite phase, resulting in some areas having an excess of carbon. • One way for carbon to leave austenite is for a phase called cementite to develop – leaving behind pure iron in the form of ferrite – producing a cementite-ferrite mixture. • Cementite, is a hard, brittle compound consisting of iron and carbon. It’s also known as iron carbide.

• The formation of pearlite involves the mixture of two phases, iron and cementite, the structure consists of alternating layers or plates. • The distance between these plates and their thickness depends on the cooling rate of the material. Fast cooling creates thin plates that are close together and slow cooling creates a much coarser structure possessing less toughness. • A fully pearlitic structure occurs at 0. 83% C, called the eutectoid point on the phase diagram.

Cast Iron • Between 2% & 4% carbon content • Standard grey cast iron very brittle

Ductile Cast iron used in drain grids
 Monopoly business examples
Monopoly business examples Steel stronger than iron
Steel stronger than iron Mass of iron in an iron tablet
Mass of iron in an iron tablet Iron sharpens iron friendship
Iron sharpens iron friendship Pure project organizational structure template
Pure project organizational structure template Pure competition market structure
Pure competition market structure Anchor plate in steel structure
Anchor plate in steel structure Flat truss examples
Flat truss examples Design philosophy of steel structure
Design philosophy of steel structure Steel structure prefab house
Steel structure prefab house Steel structure design
Steel structure design Steel structure design
Steel structure design Prefabricated buildings hs code
Prefabricated buildings hs code Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html



























