The Structure of an Atom Chapter 4 Daltons








- Slides: 41

The Structure of an Atom Chapter 4

Dalton’s Atomic Theory • 1. All elements are made up of tiny indivisible particles • 2. Atoms of the same element are identical. • 3. Atoms of different elements can mix to form compounds in simple whole # ratios • 4. Chemical reactions occur when atoms are separated and rearrange.

How small is an atom? IF YOU PLACED 100, 000 COPPER ATOMS SIDE BY SIDE, THE LINE WOULD BE 1 cm LONG

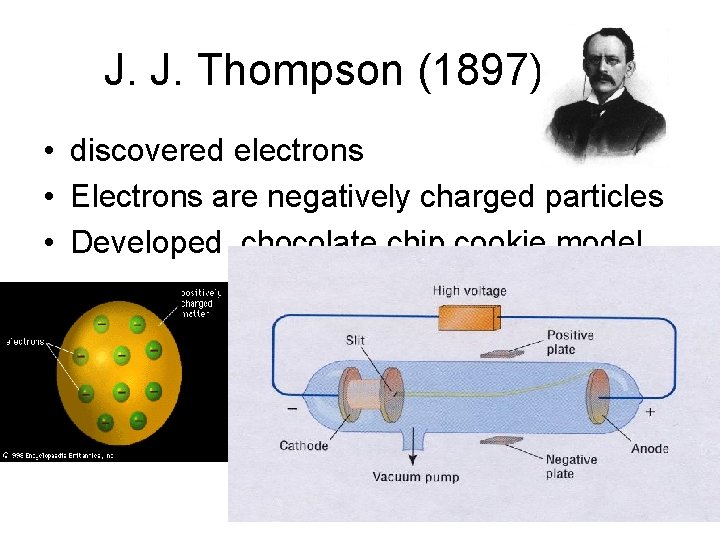
J. J. Thompson (1897) • discovered electrons • Electrons are negatively charged particles • Developed chocolate chip cookie model

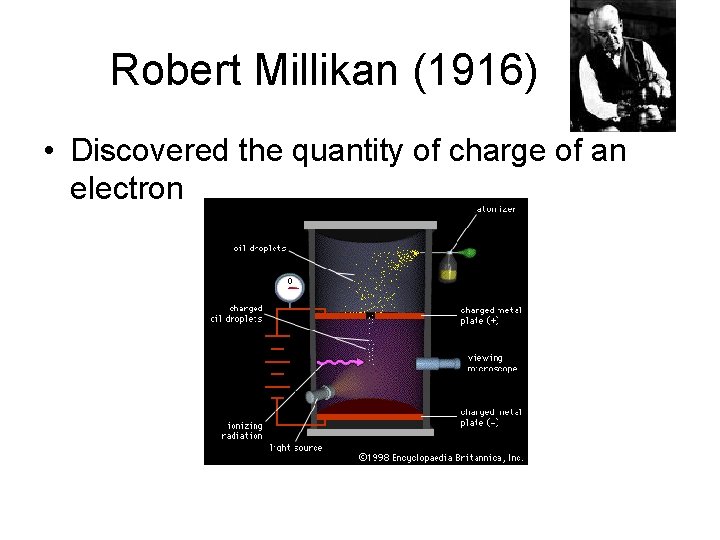
Robert Millikan (1916) • Discovered the quantity of charge of an electron

Protons & Neutrons • E. Goldstein (1886)– discovered protons – Protons are positively charged particles • James Chadwick (1932)– discovered neutrons – Neutrons are particles without a charge

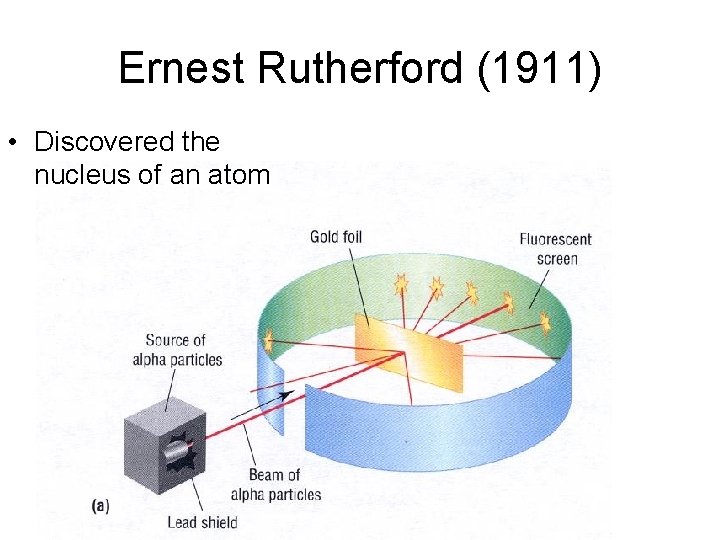
Ernest Rutherford (1911) • Discovered the nucleus of an atom

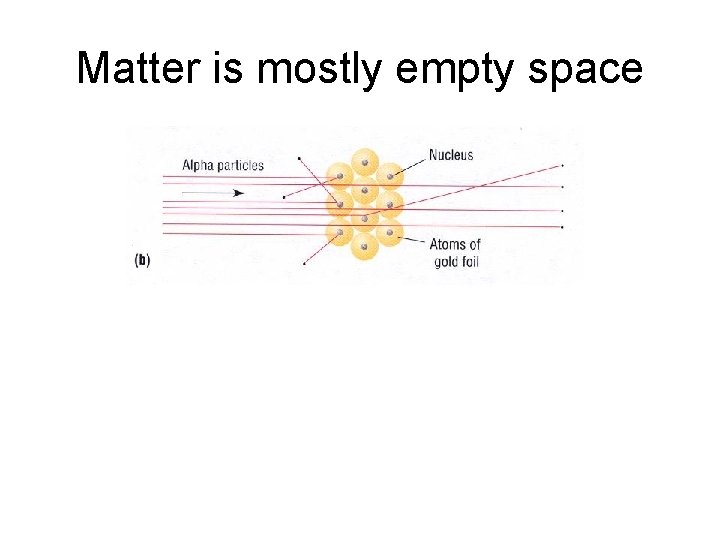
Matter is mostly empty space

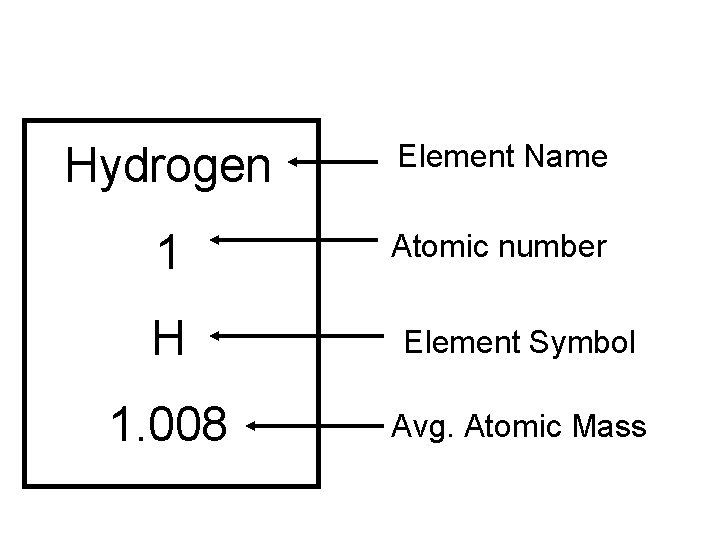
Hydrogen Element Name 1 Atomic number H Element Symbol 1. 008 Avg. Atomic Mass

Atomic Structure Notes EQ: How do Atoms of the Same Element Differ?

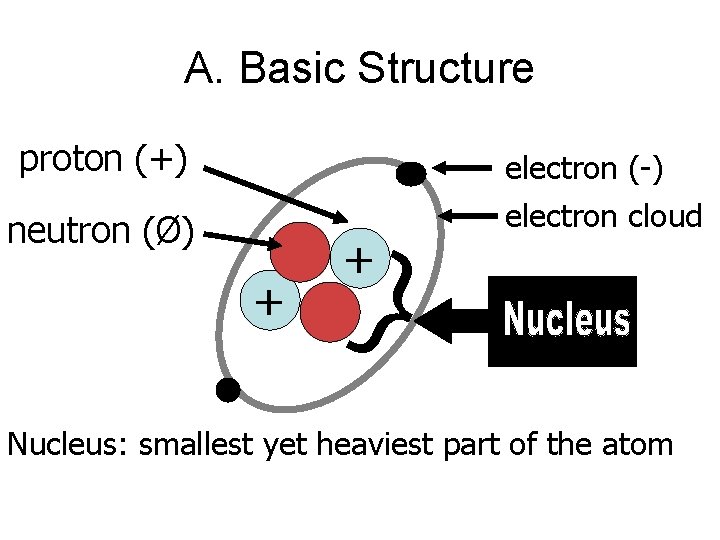
A. Basic Structure proton (+) neutron (Ø) + } + electron (-) electron cloud Nucleus: smallest yet heaviest part of the atom

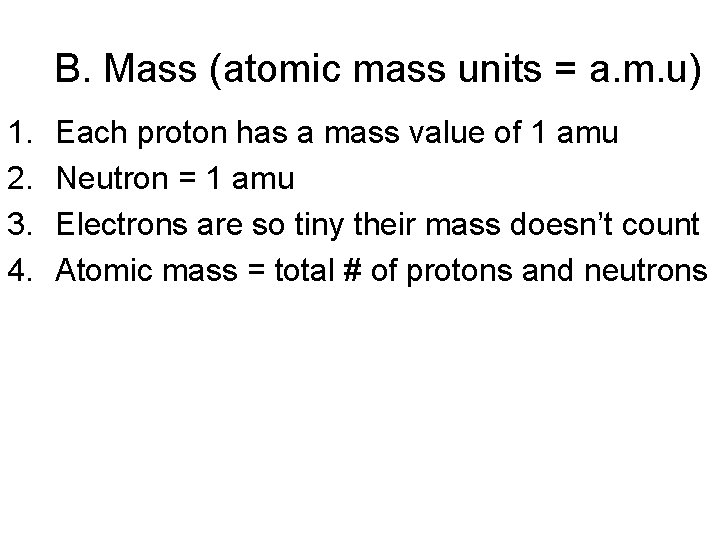
B. Mass (atomic mass units = a. m. u) 1. 2. 3. 4. Each proton has a mass value of 1 amu Neutron = 1 amu Electrons are so tiny their mass doesn’t count Atomic mass = total # of protons and neutrons

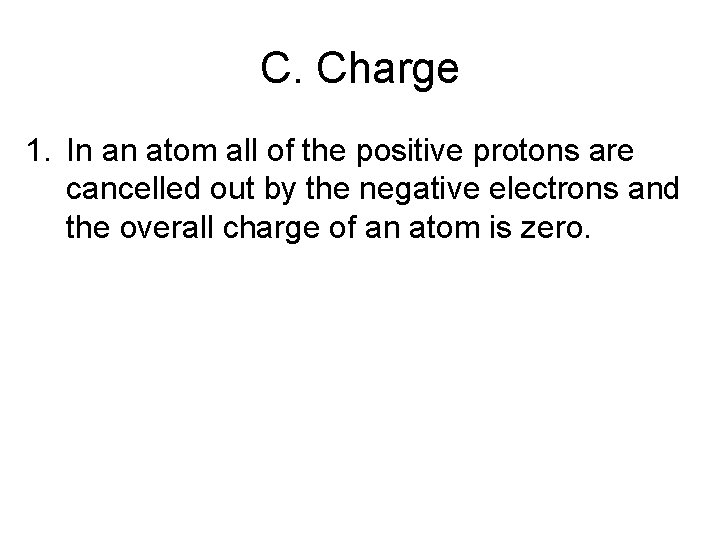
C. Charge 1. In an atom all of the positive protons are cancelled out by the negative electrons and the overall charge of an atom is zero.

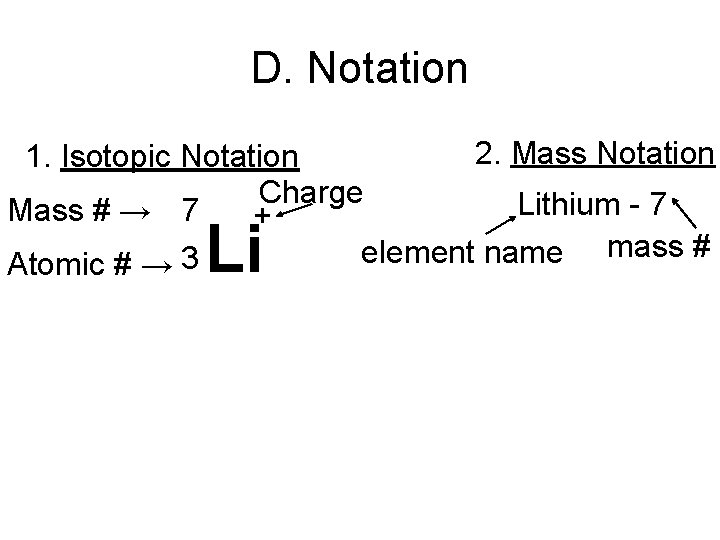
D. Notation 2. Mass Notation 1. Isotopic Notation Charge Lithium - 7 Mass # → 7 + mass # element name 3 Atomic # → Li

E. Atomic Number 1. Atomic # = # Protons 2. # electrons = # protons when atom is neutral

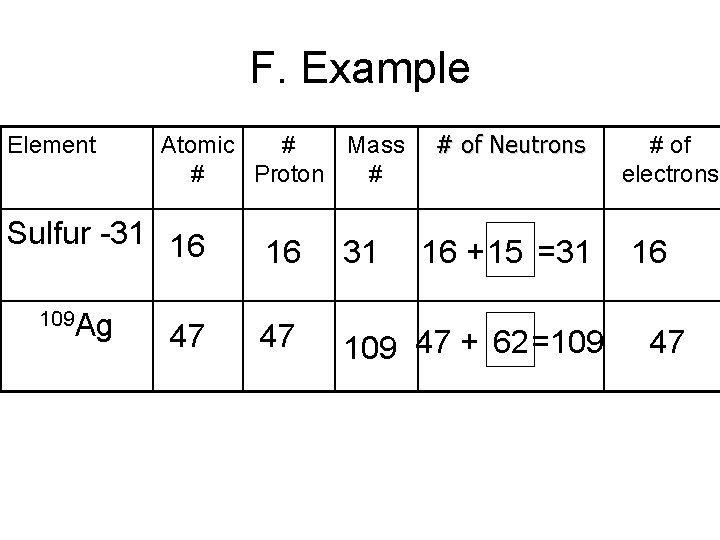
F. Example Element Atomic # Mass # Proton # Sulfur -31 16 109 Ag 47 # of Neutrons 16 31 16 + 15 =31 47 109 47 + 62 =109 # of electrons 16 47

Isotope Notes EQ: What is an isotope?

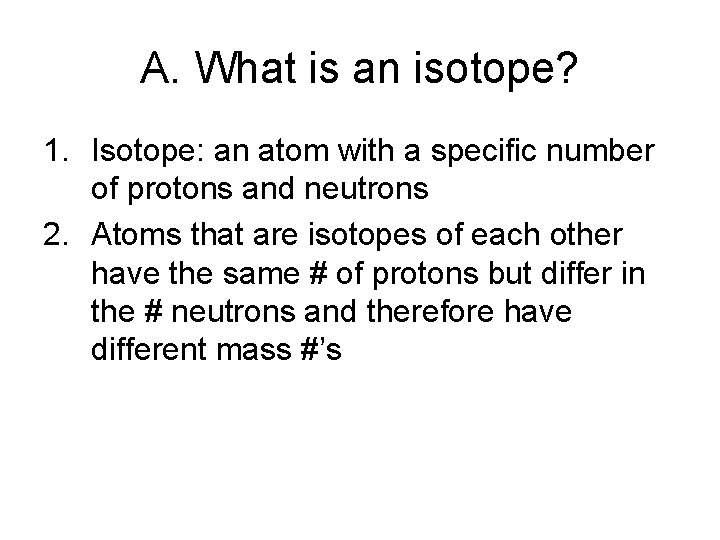
A. What is an isotope? 1. Isotope: an atom with a specific number of protons and neutrons 2. Atoms that are isotopes of each other have the same # of protons but differ in the # neutrons and therefore have different mass #’s

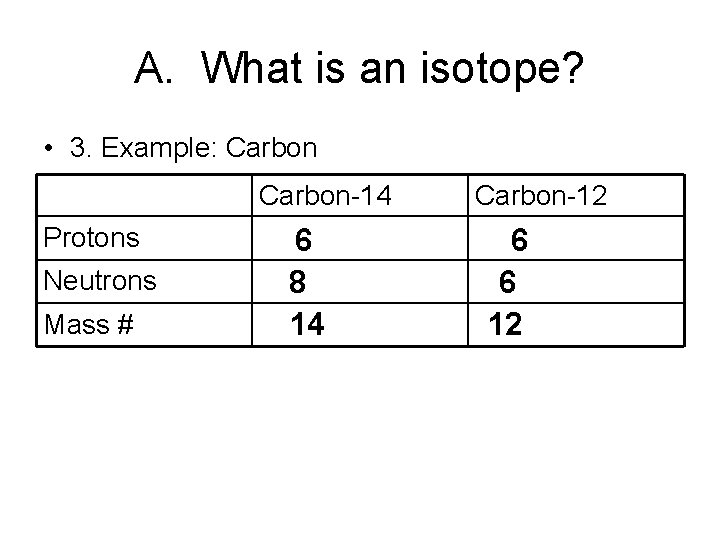
A. What is an isotope? • 3. Example: Carbon-14 Protons Neutrons Mass # 6 8 14 Carbon-12 6 6 12

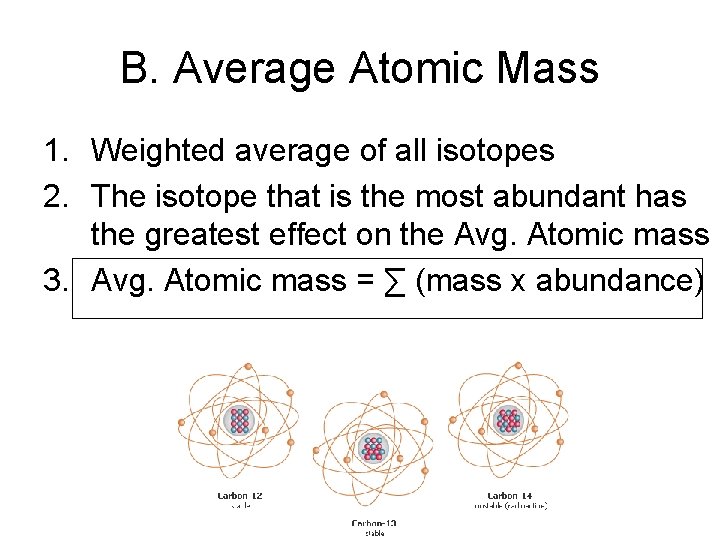
B. Average Atomic Mass 1. Weighted average of all isotopes 2. The isotope that is the most abundant has the greatest effect on the Avg. Atomic mass 3. Avg. Atomic mass = ∑ (mass x abundance)

• Ex 1: There are two isotopes of copper. Cu 63 and Cu-65. Which is the most abundant? Cu-63 b/c the mass is closer to the avg. atomic mass

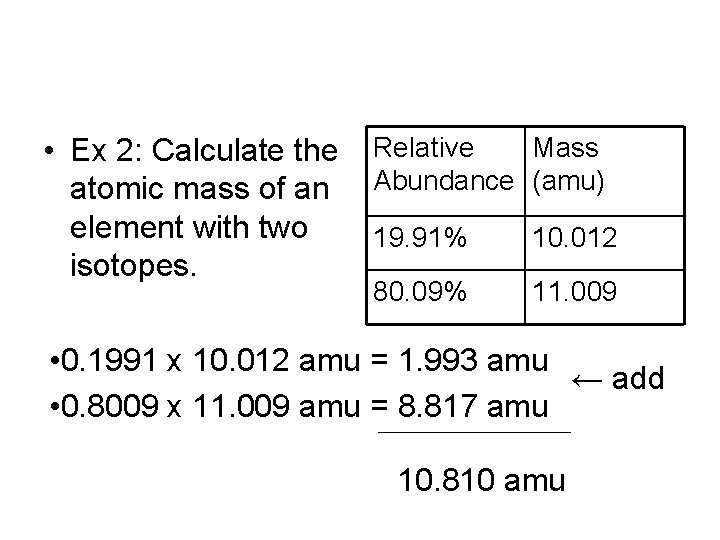
• Ex 2: Calculate the atomic mass of an element with two isotopes. Relative Mass Abundance (amu) 19. 91% 10. 012 80. 09% 11. 009 • 0. 1991 x 10. 012 amu = 1. 993 amu ← add • 0. 8009 x 11. 009 amu = 8. 817 amu 10. 810 amu

Ion Notes EQ: How are ions and atoms different?

A. Definitions 1. Ion: When an atom gains or loses an electron, it has an imbalance of charge a) Cation (+) = loses e- to become positive b) Anion (-) = gains e- to become negative 2. In an ion, the number of protons do not equal the number of electrons

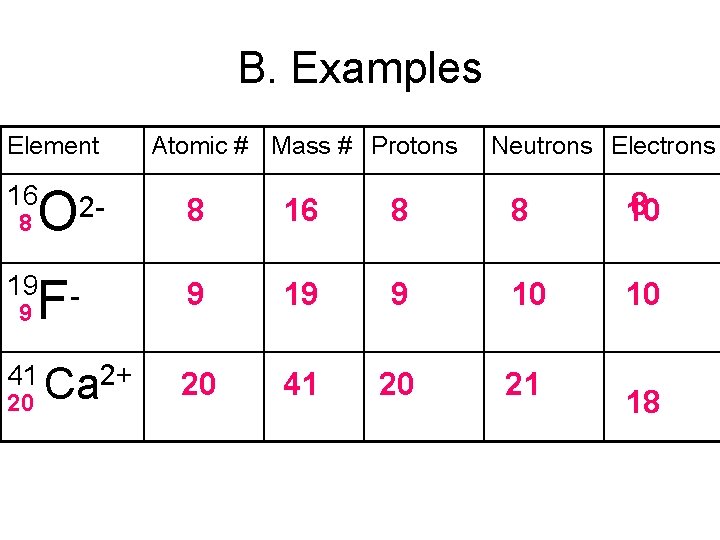
B. Examples Element 16 O Atomic # Mass # Protons Neutrons Electrons 8 16 8 8 8 10 19 9 10 10 41 Ca 2+ 20 41 20 21 8 2 - F 9 20 18

Unstable Nuclei

A. Normal Reactions 1. atoms rearrange, the elements do not change

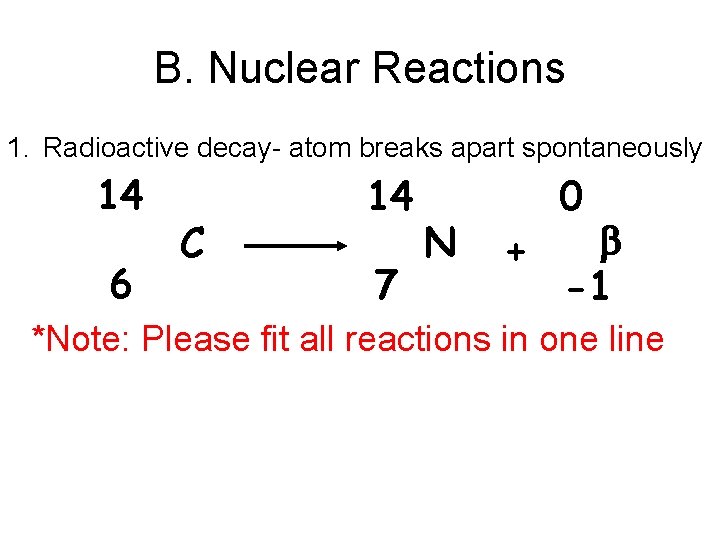
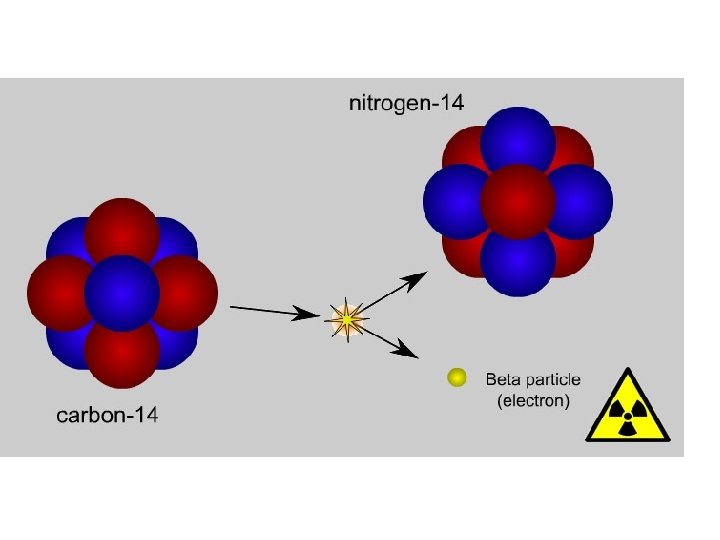
B. Nuclear Reactions 1. Radioactive decay- atom breaks apart spontaneously 14 6 C 14 7 N + 0 -1 *Note: Please fit all reactions in one line


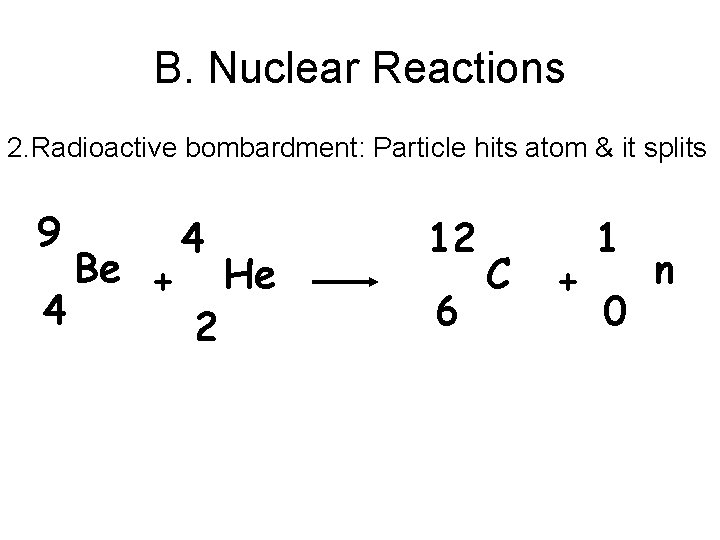
B. Nuclear Reactions 2. Radioactive bombardment: Particle hits atom & it splits 9 4 Be + 4 2 He 12 6 C + 1 0 n


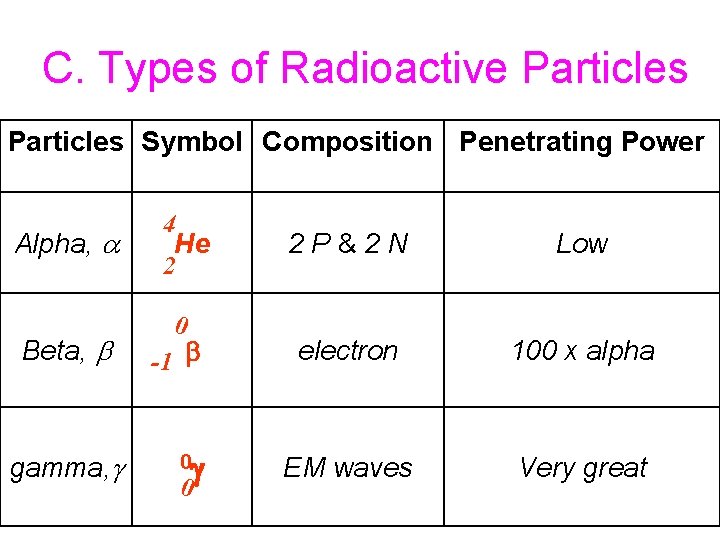
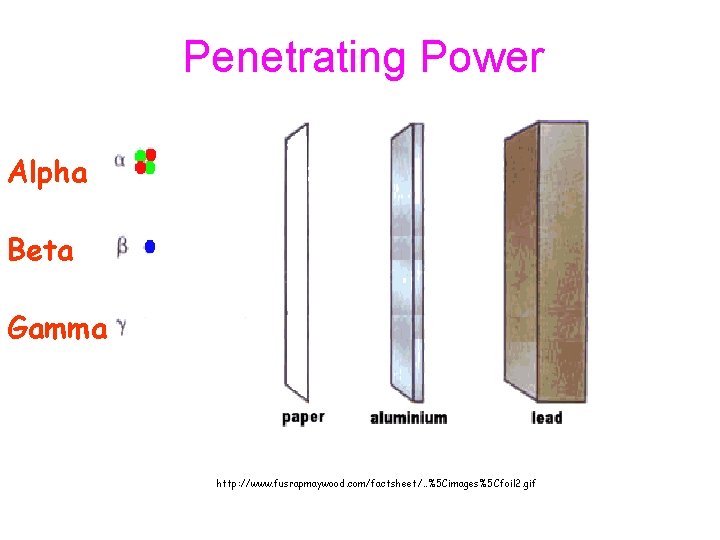
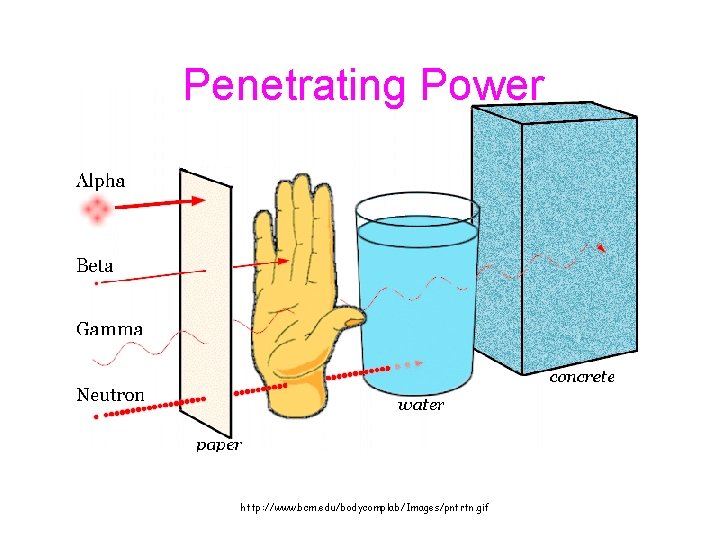
C. Types of Radioactive Particles Symbol Composition Penetrating Power Alpha, Beta, gamma, 4 He 2 0 -1 0 0 2 P&2 N Low electron 100 x alpha EM waves Very great

Penetrating Power Alpha Beta Gamma http: //www. fusrapmaywood. com/factsheet/. . %5 Cimages%5 Cfoil 2. gif

Penetrating Power http: //www. bcm. edu/bodycomplab/Images/pntrtn. gif

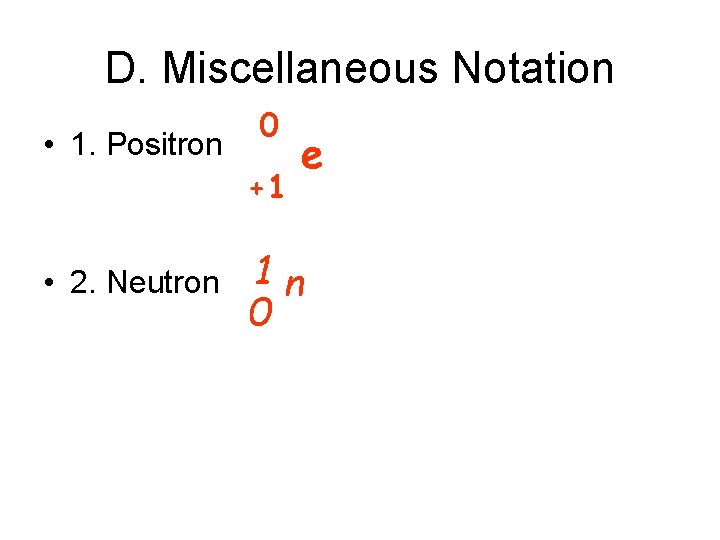
D. Miscellaneous Notation • 1. Positron 0 +1 • 2. Neutron e 1 n 0

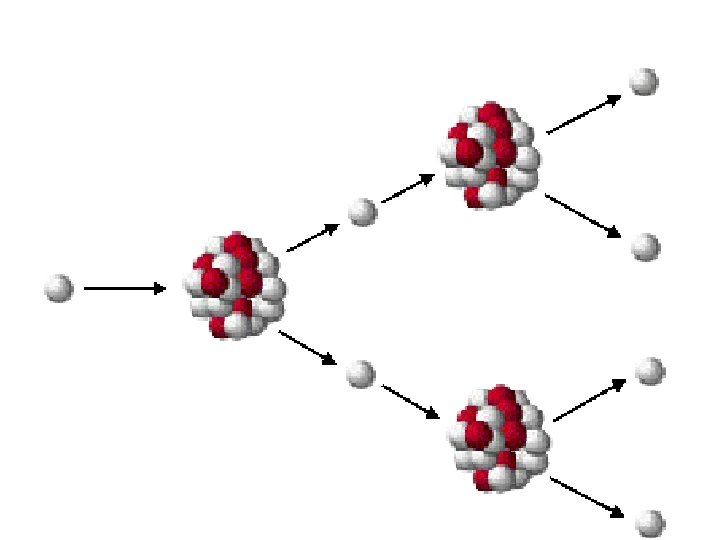
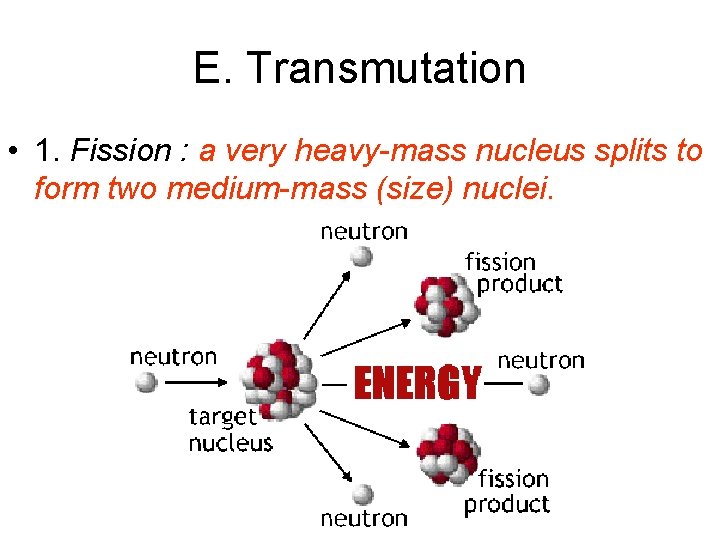
E. Transmutation • 1. Fission : a very heavy-mass nucleus splits to form two medium-mass (size) nuclei.

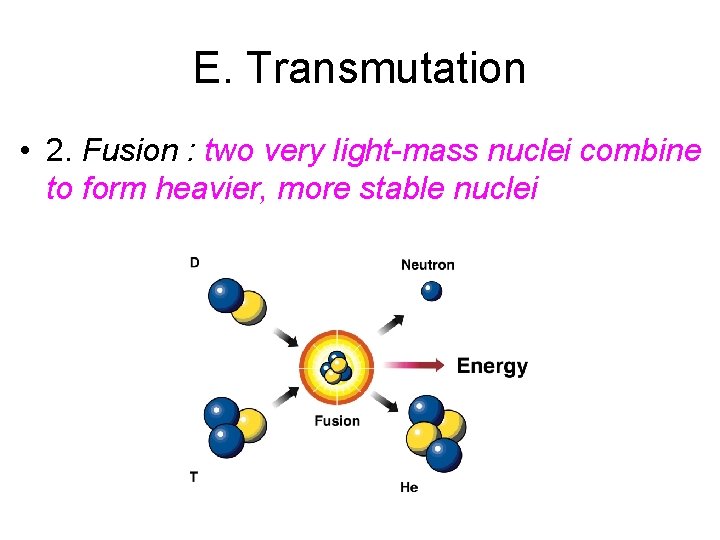
E. Transmutation • 2. Fusion : two very light-mass nuclei combine to form heavier, more stable nuclei

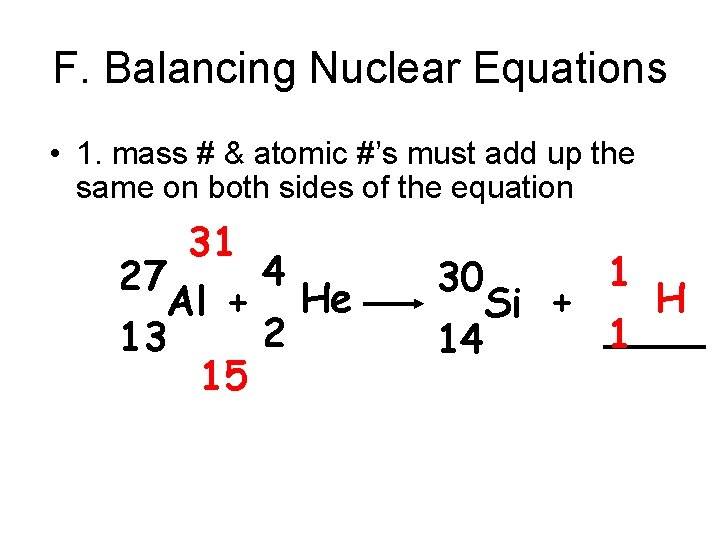
F. Balancing Nuclear Equations • 1. mass # & atomic #’s must add up the same on both sides of the equation 31 4 27 Al + He 2 13 15 1 30 H Si + ____ 1 14

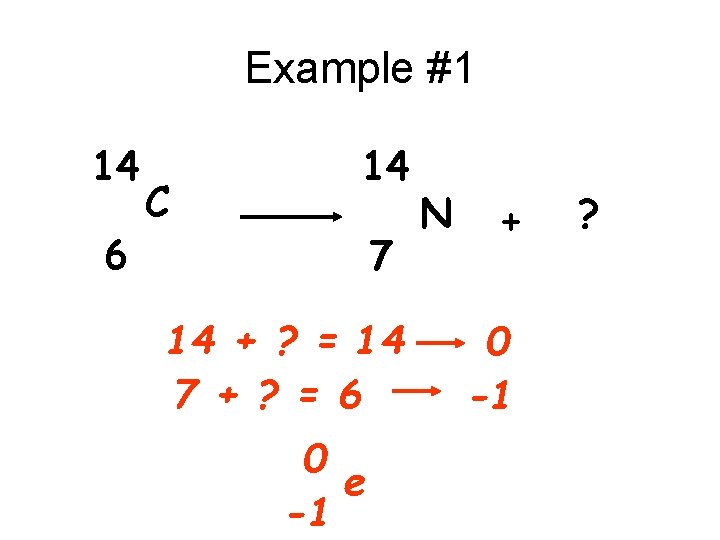
Example #1 14 6 C 14 7 14 + ? = 14 7 + ? = 6 0 e -1 N + 0 -1 ?

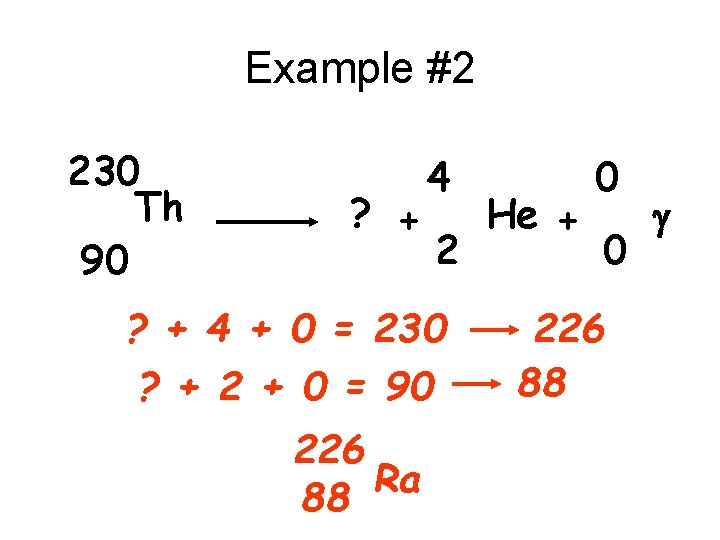
Example #2 230 Th 90 ? + 4 2 ? + 4 + 0 = 230 ? + 2 + 0 = 90 226 Ra 88 He + 0 0 226 88

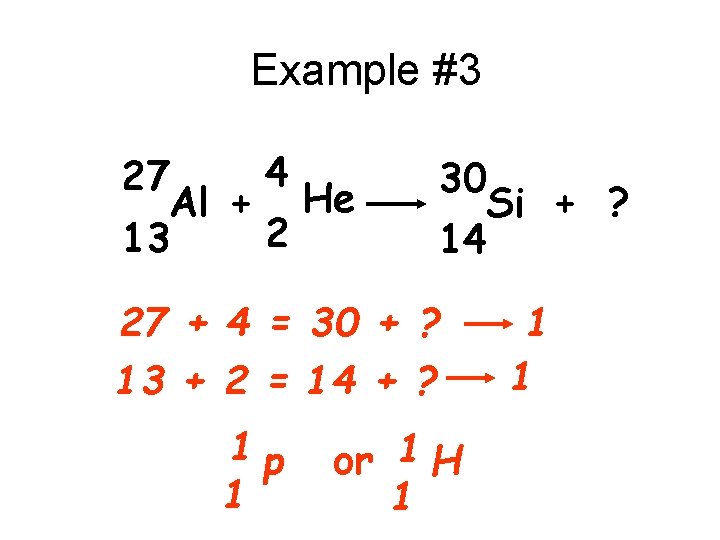
Example #3 4 27 Al + He 2 13 30 Si + ? 14 27 + 4 = 30 + ? 13 + 2 = 14 + ? 1 p 1 or 1 H 1 1 1