THE STRUCTURE FUNCTION OF LARGE BIOLOGICAL MACROMOLECULES Campbell
THE STRUCTURE & FUNCTION OF LARGE BIOLOGICAL MACROMOLECULES Campbell and Reece CHAPTER 5
Macromolecules are Polymers polymer: long molecule consisting of many similar, sometimes identical, building blocks linked by covalent bonds monomer: the smaller units that make up a polymer
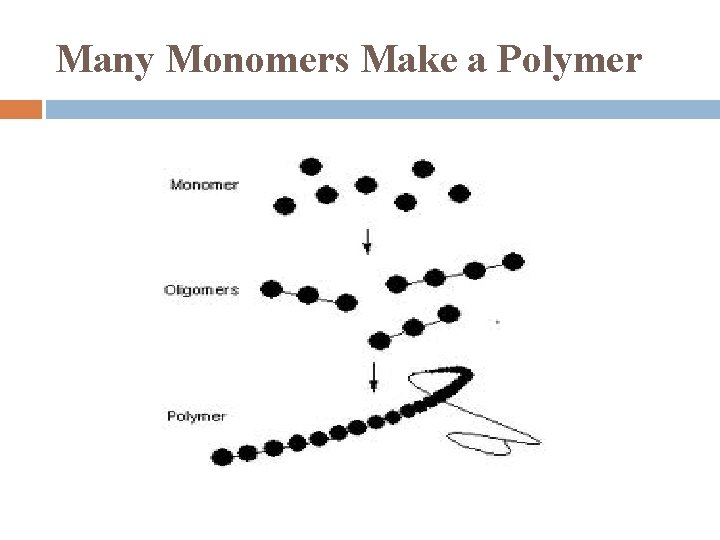
Many Monomers Make a Polymer
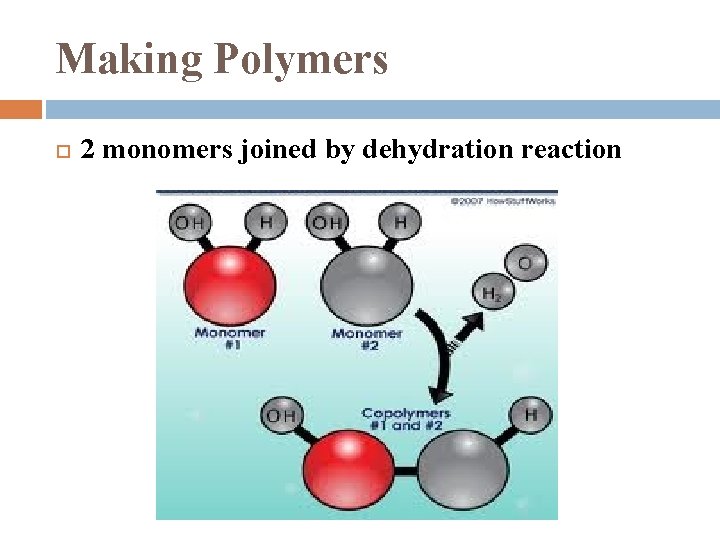
Making Polymers 2 monomers joined by dehydration reaction
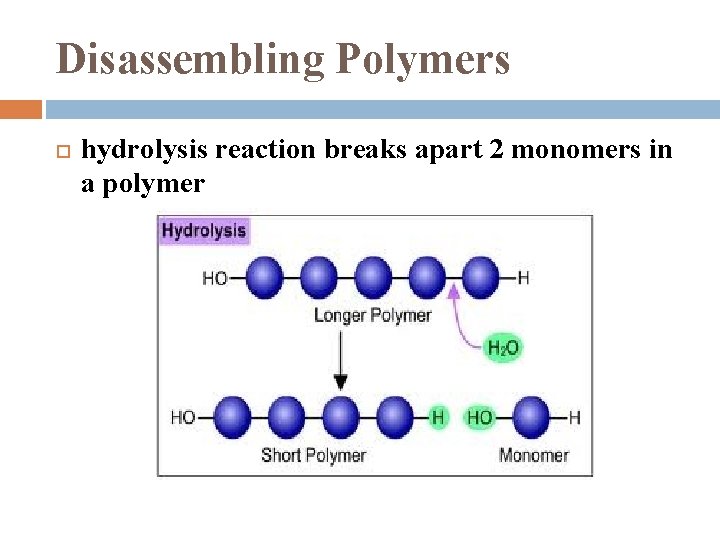
Disassembling Polymers hydrolysis reaction breaks apart 2 monomers in a polymer
Diversity of Polymers possible varieties of macromolecules infinite only use 40 -50 monomers small molecules common to all organisms are ordered into species unique macromolecules

Carbohydrates Simple Carbohydrates � Sugars � Monosaccharides � Disaccharides Complex Carbohydrates � Polysaccharides
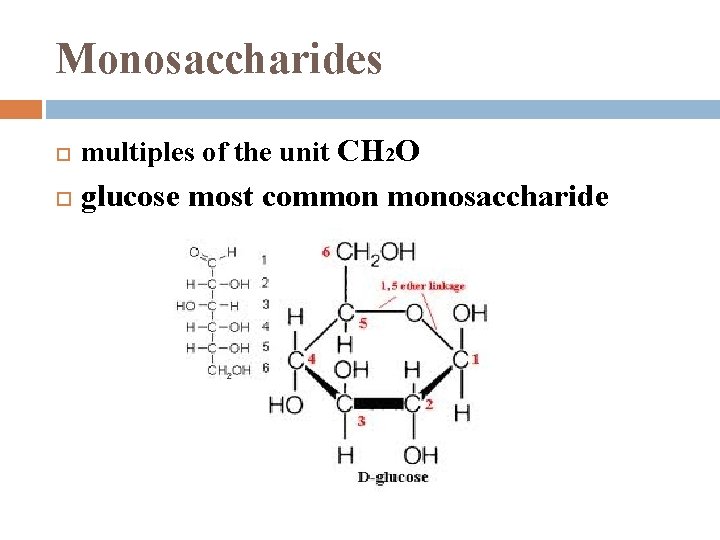
Monosaccharides multiples of the unit CH 2 O glucose most common monosaccharide
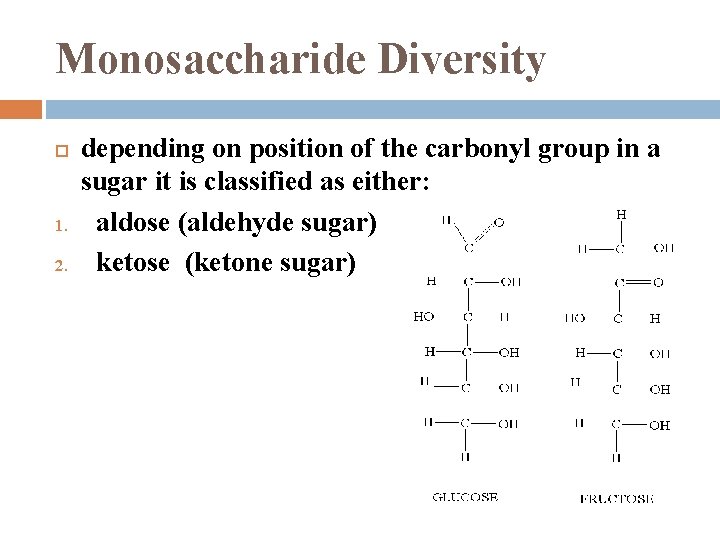
Monosaccharide Diversity 1. 2. depending on position of the carbonyl group in a sugar it is classified as either: aldose (aldehyde sugar) ketose (ketone sugar)
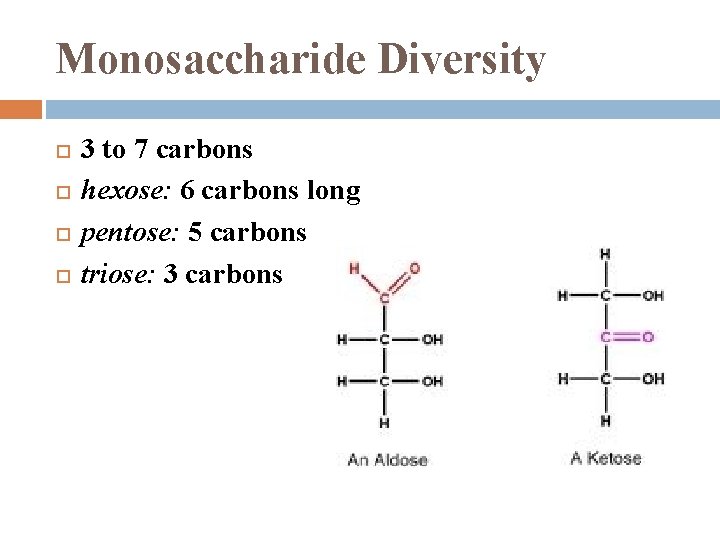
Monosaccharide Diversity 3 to 7 carbons hexose: 6 carbons long pentose: 5 carbons triose: 3 carbons
Monosaccharide Diversity most hexoses and pentoses form rings in aqueous solutions used in cellular respiration (especially glucose) serve as raw materials for synthesis of amino acids and fatty acids � if not immediately used in these ways used to build disaccharides or polysaccharides
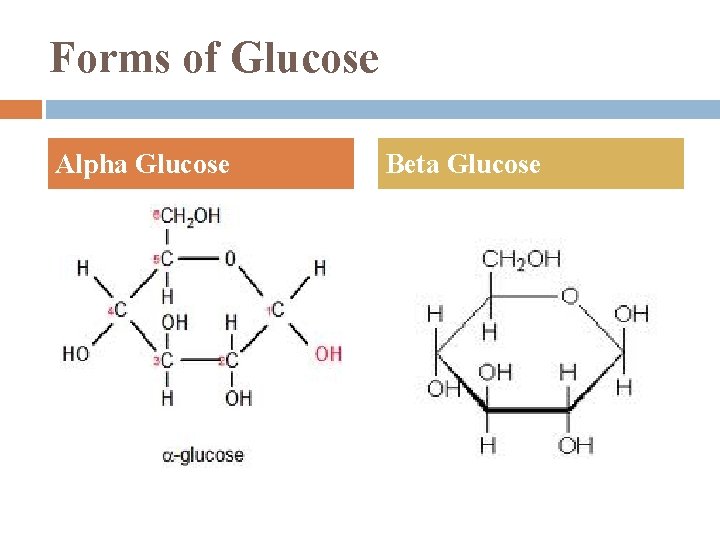
Forms of Glucose Alpha Glucose Beta Glucose
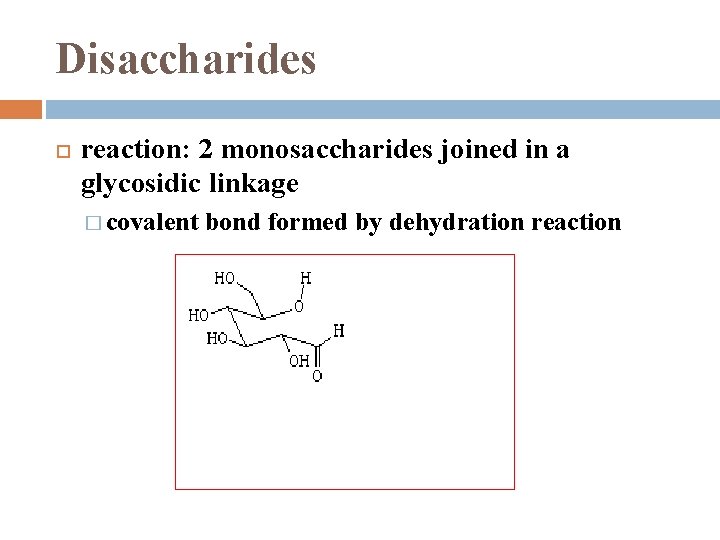
Disaccharides reaction: 2 monosaccharides joined in a glycosidic linkage � covalent bond formed by dehydration reaction
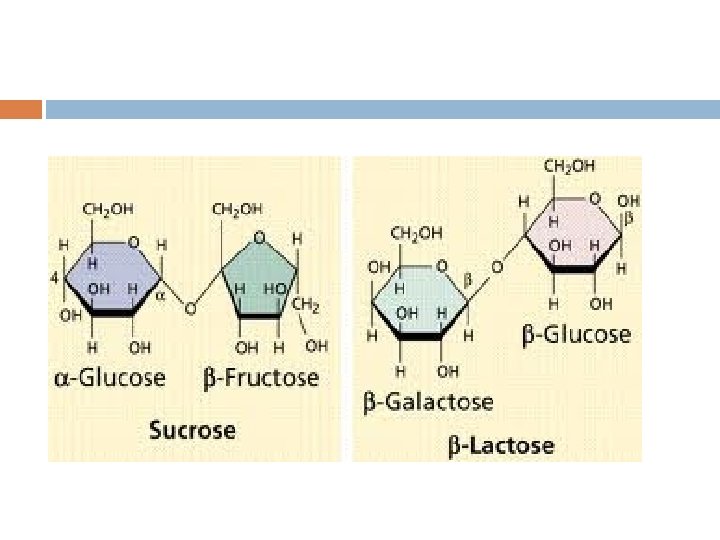
Disaccharides 2 glucose = maltose (malt sugar) glucose + galactose glucose + fructose = sucrose (table sugar) sucrose: form plants use to transport sugars from leaves roots & other nonphotosynthetic parts of plant
Polysaccharides 1. 2. polymers of hundreds to thousands of monosaccharides joined by glycosidic linkages function determined by its sugar monomers & positions of glycosidic linkages 2 types: storage of monosaccharides to be used for energy when needed building material
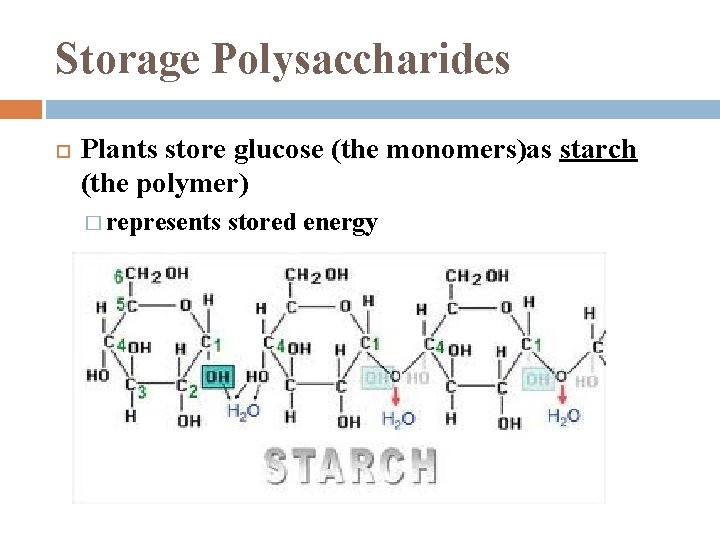
Storage Polysaccharides Plants store glucose (the monomers)as starch (the polymer) � represents stored energy

Starch most is made of α glucose monomers joined in 14 linkages � simplest form of starch (amylose) is unbranched complex starch, amylopectin, has 1 -6 linkage

Storage Polysaccharides Animals: store glucose (the monomers) as glycogen (the polymer) in 1 -4 & 1 -6 linkages � stored mainly in liver & muscle cells � humans store about 1 days supply of glucose this way
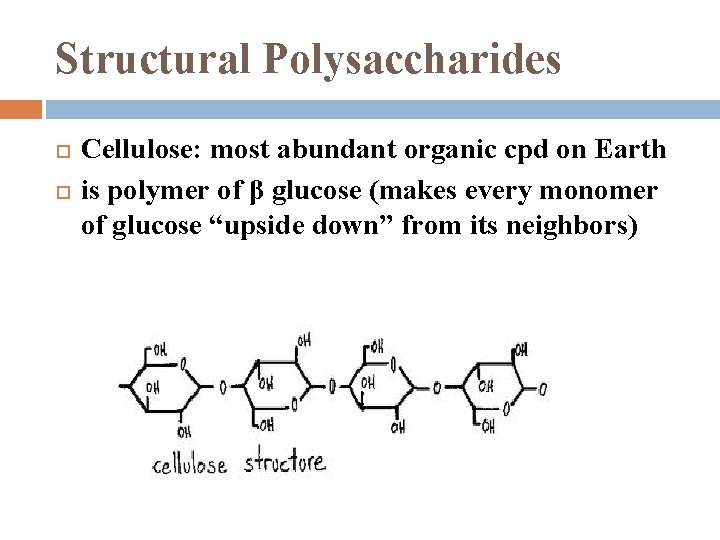
Structural Polysaccharides Cellulose: most abundant organic cpd on Earth is polymer of β glucose (makes every monomer of glucose “upside down” from its neighbors)
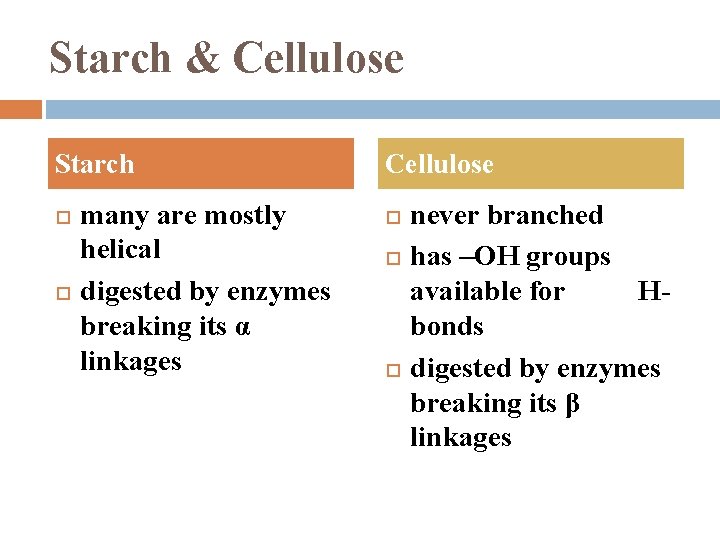
Starch & Cellulose Starch many are mostly helical digested by enzymes breaking its α linkages Cellulose never branched has –OH groups available for Hbonds digested by enzymes breaking its β linkages

Cellulose digested by very few organisms (don’t have enzymes to do it) in humans: passes thru GI tract abrading walls & stimulating mucus secretion along the way smoother passage of food thru not technically a nutrient but is important

“Insoluble Fiber” = Cellulose

Cellulose Cows: have bacteria and protists in their guts that have enzymes that can digest cellulose nutrients that can be used by cow Termites unable to digest cellulose in wood it eats have prokaryotes & protists to break it down and so termite can use nutrients
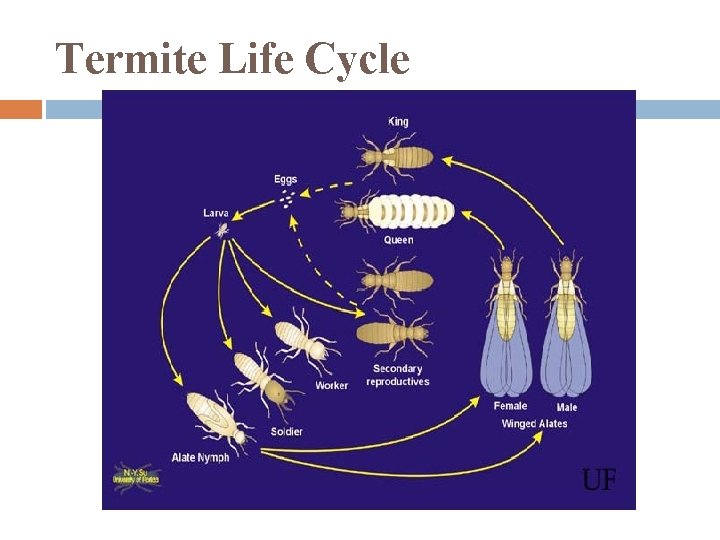
Termite Life Cycle

Termites

Chitin another structural polysaccharide used by arthropods to build exoskeletons: made of chitin + calcium carbonate
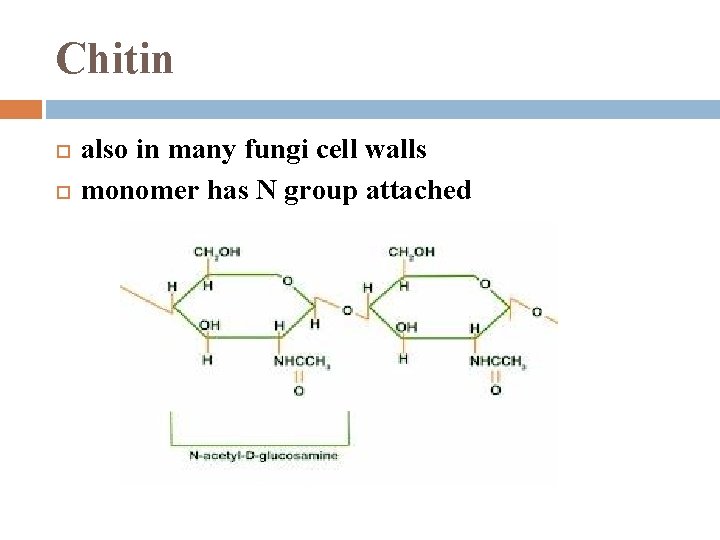
Chitin also in many fungi cell walls monomer has N group attached


Lipids large group of hydrophobic molecules do not have true monomers Includes: � Waxes � Steroids � Some Pigments � Oils, Fats � Phospholipids
Fats 1. 2. large molecules assembled from smaller molecules by a dehydration reaction 2 parts: Glycerol Fatty Acid
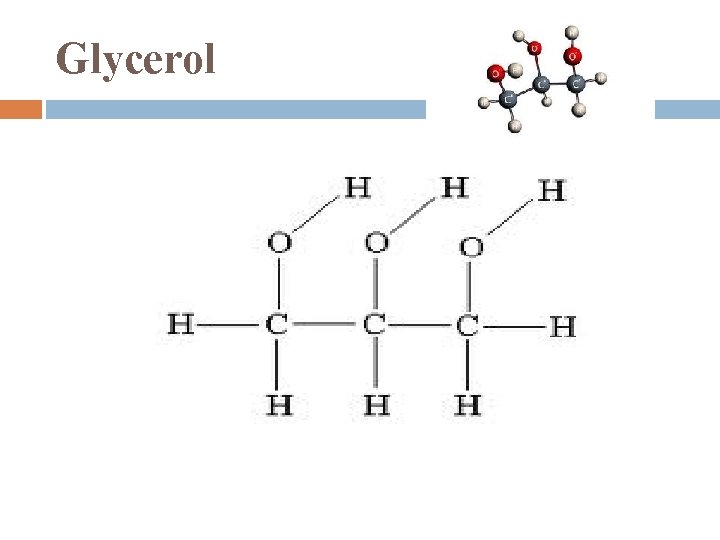
Glycerol
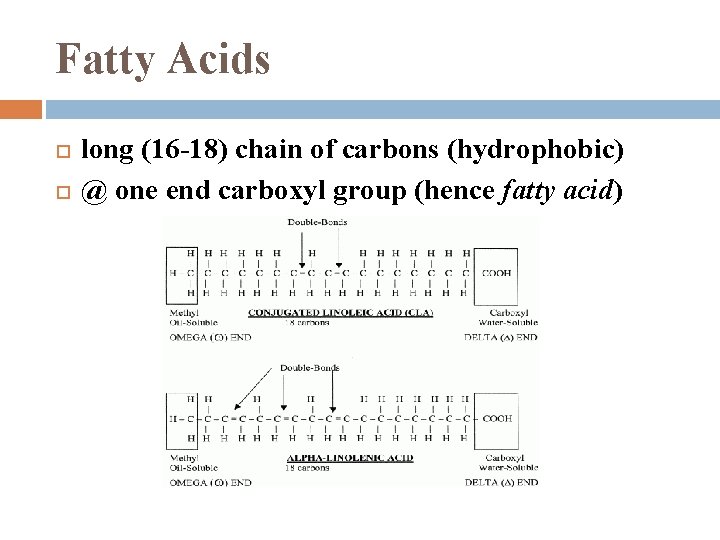
Fatty Acids long (16 -18) chain of carbons (hydrophobic) @ one end carboxyl group (hence fatty acid)

Triglyceride 3 fatty acids + glycerol
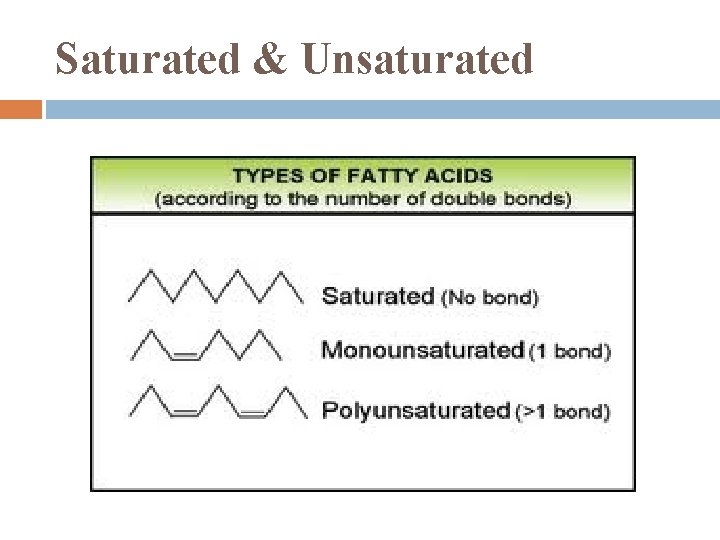
Saturated & Unsaturated
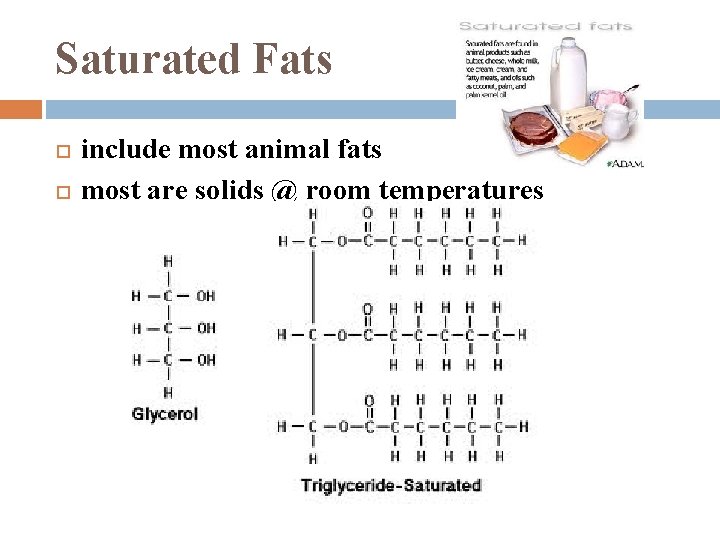
Saturated Fats include most animal fats most are solids @ room temperatures
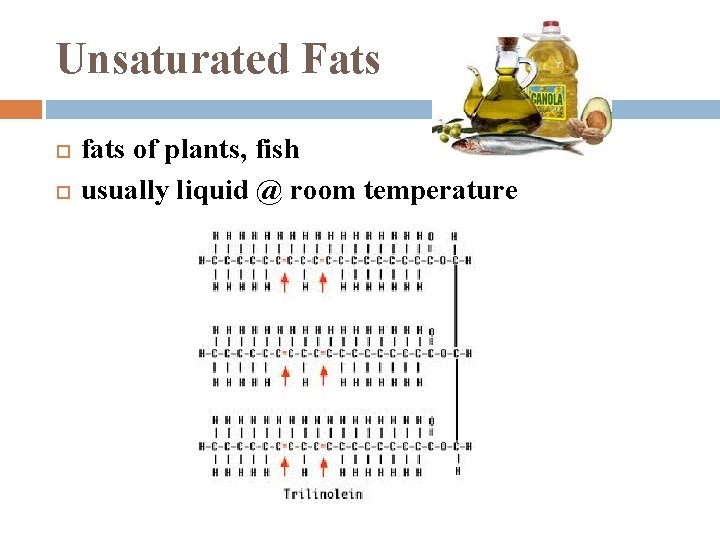
Unsaturated Fats fats of plants, fish usually liquid @ room temperature

Hydrogenated Vegetable Oil seen on some food labels means that unsaturated fats have been synthetically converted to saturated fats to keep from separating
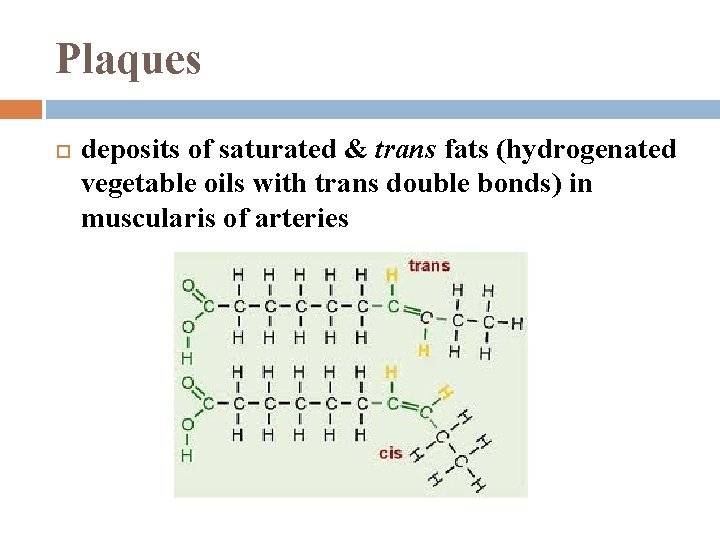
Plaques deposits of saturated & trans fats (hydrogenated vegetable oils with trans double bonds) in muscularis of arteries
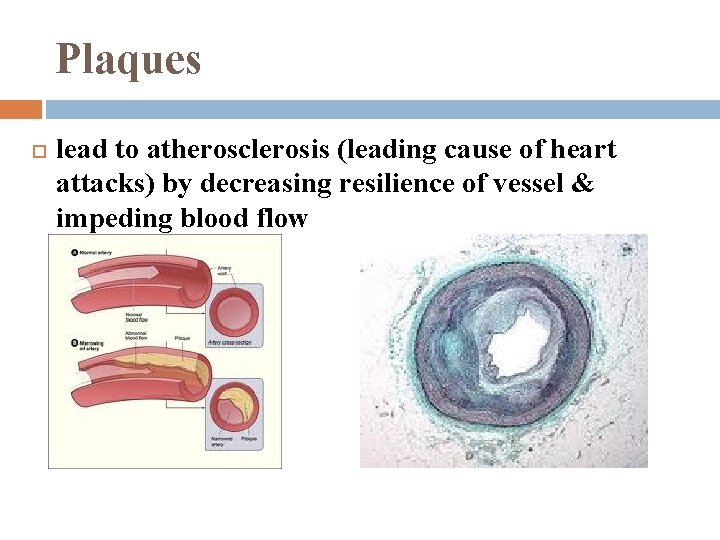
Plaques lead to atherosclerosis (leading cause of heart attacks) by decreasing resilience of vessel & impeding blood flow

Trans Fats USDA now requires nutritional labels to include amount of trans fats some cities & Denmark ban restaurants from using trans fats

Essential Fatty Acids cannot be synthesized in body so must be included in diet include: omega-3 fatty acids: required for normal growth in children probably protect against cardiovascular disease in adults
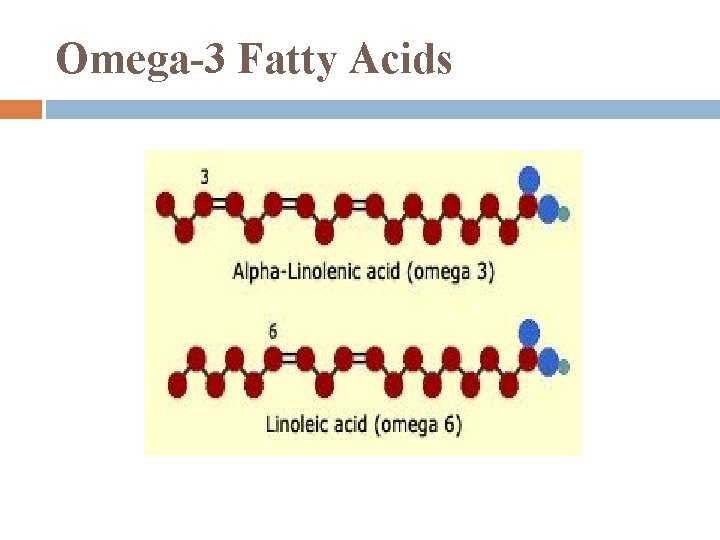
Omega-3 Fatty Acids

Energy Storage 1 g fat has 2 x chemical potential energy as 1 g of polysaccharide plants (generally immobile) can store majority of their energy in polysaccharides except vegetable oils extracted from their seeds
Functions of Fat 1. 2. 3. Plants: storage of energy Animals: storage of energy protect organs insulation

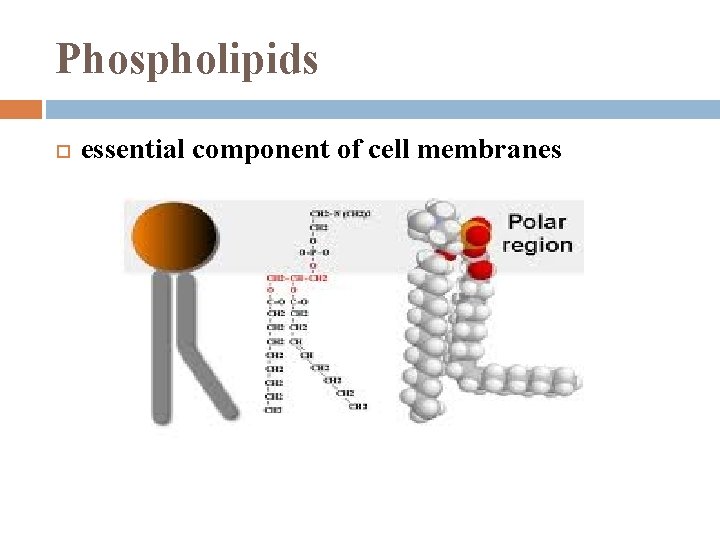
Phospholipids essential component of cell membranes
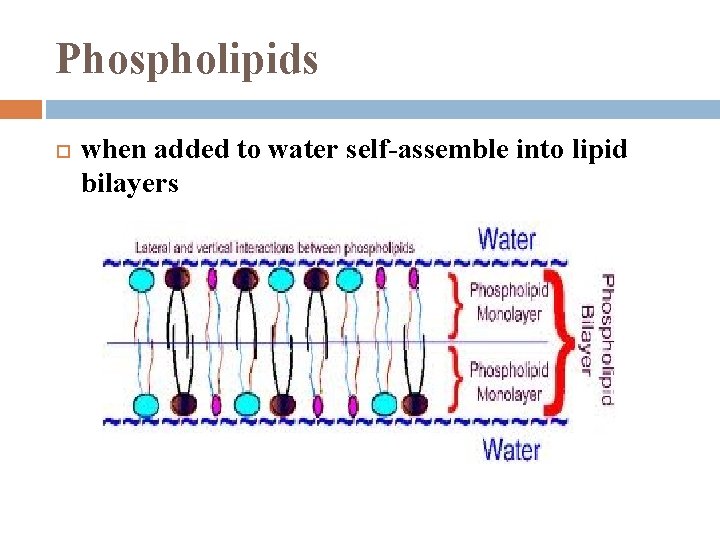
Phospholipids when added to water self-assemble into lipid bilayers
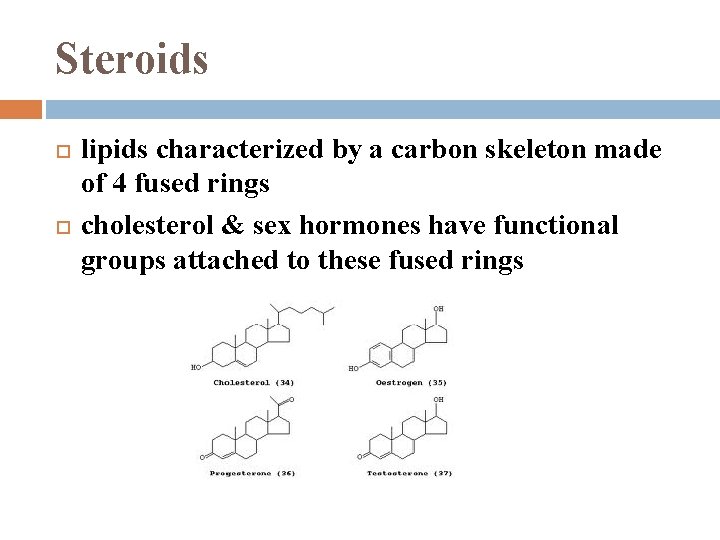
Steroids lipids characterized by a carbon skeleton made of 4 fused rings cholesterol & sex hormones have functional groups attached to these fused rings
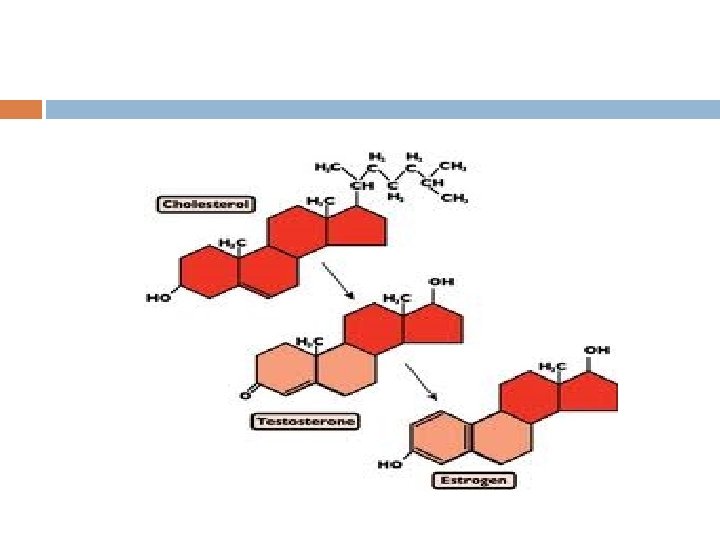
Cholesterol in Animals part of cell membranes precursor for other steroids vertebrates make it in liver + dietary intake saturated fats & trans fats increase cholesterol levels which is ass’c with atherosclerotic disease

In plant seeds, the inside of the seed is rich in lipids (oils). Describe & explain the form the membrane around a droplet of oil would need to take:


Proteins word in Greek from “primary” account for >50% of dry mass of most cells instrumental in almost everything organisms do
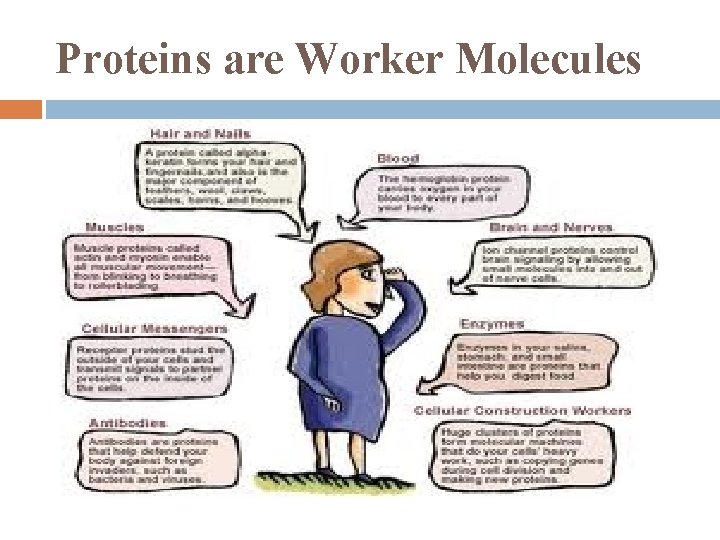
Proteins are Worker Molecules
Proteins humans have tens of thousands of proteins, each with specific structure & function all made from 20 amino acids (a. a. ) Proteins are biologically functional molecules made of 1 or more polypeptides, each folded & coiled into a specific 3 -D structure
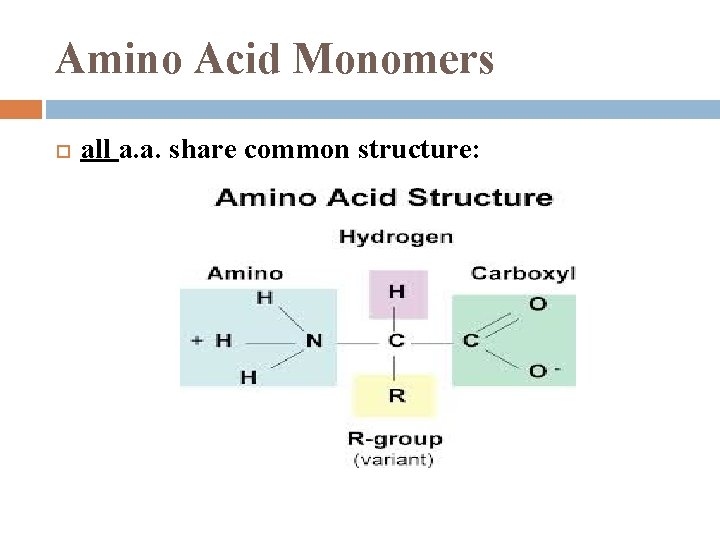
Amino Acid Monomers all a. a. share common structure:
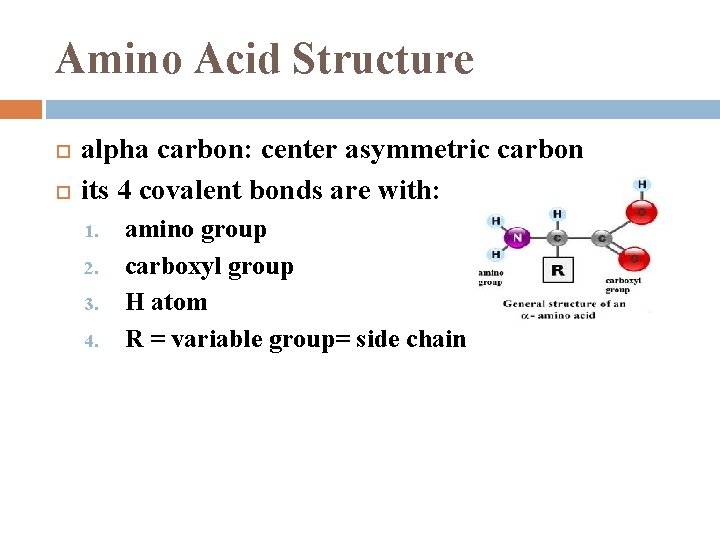
Amino Acid Structure alpha carbon: center asymmetric carbon its 4 covalent bonds are with: 1. 2. 3. 4. amino group carboxyl group H atom R = variable group= side chain
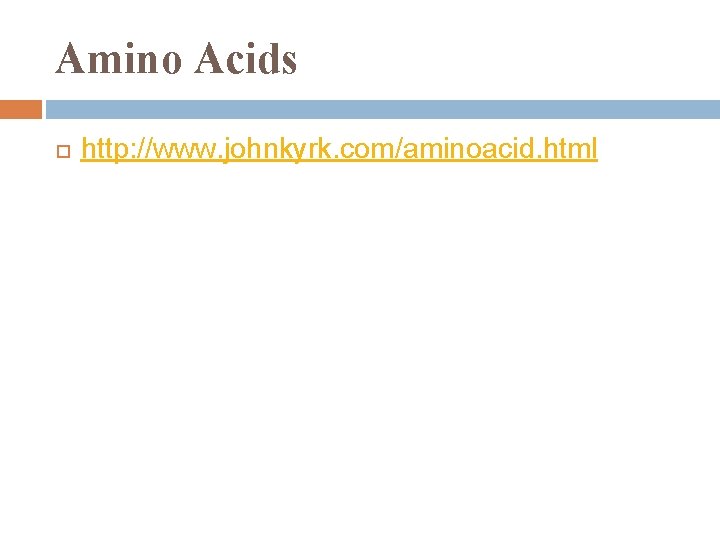
Amino Acids http: //www. johnkyrk. com/aminoacid. html
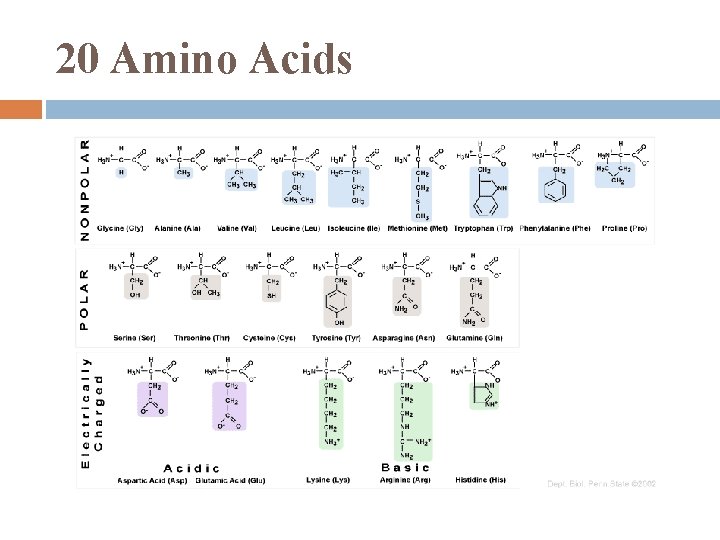
20 Amino Acids
R Groups its physical & chemical properties determine the unique characteristics of a. a. so affect the physical & chemical properties of the polypeptide chain
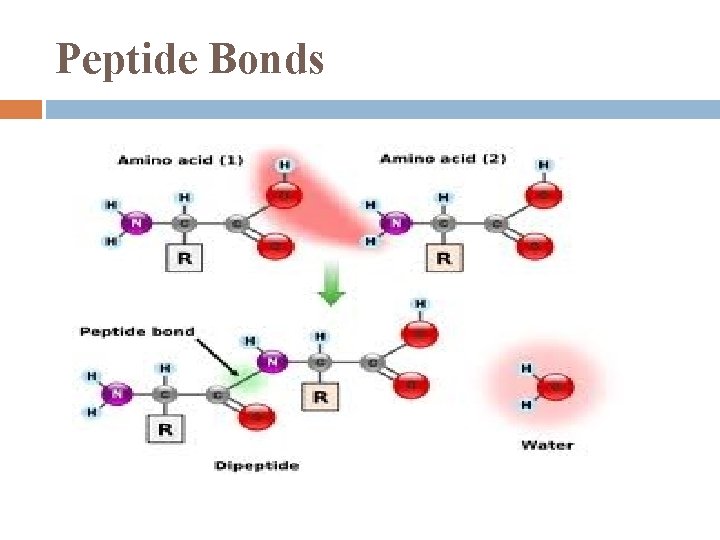
Peptide Bonds
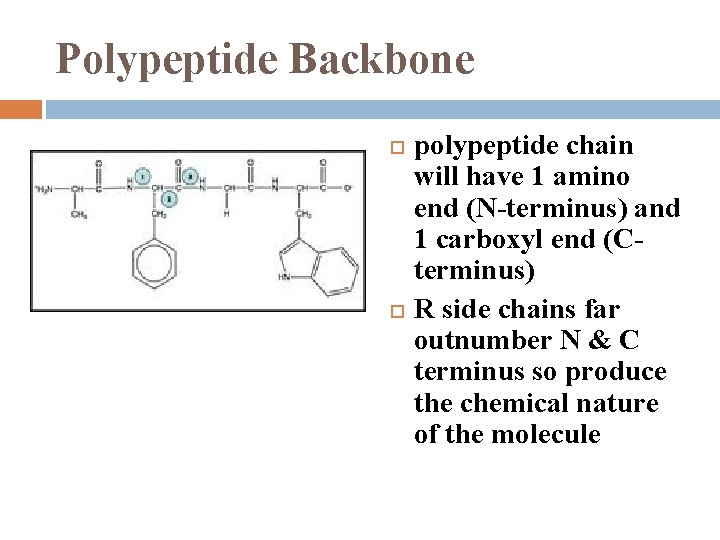
Polypeptide Backbone polypeptide chain will have 1 amino end (N-terminus) and 1 carboxyl end (Cterminus) R side chains far outnumber N & C terminus so produce the chemical nature of the molecule
Protein Structure & Function polypeptide ≠ protein
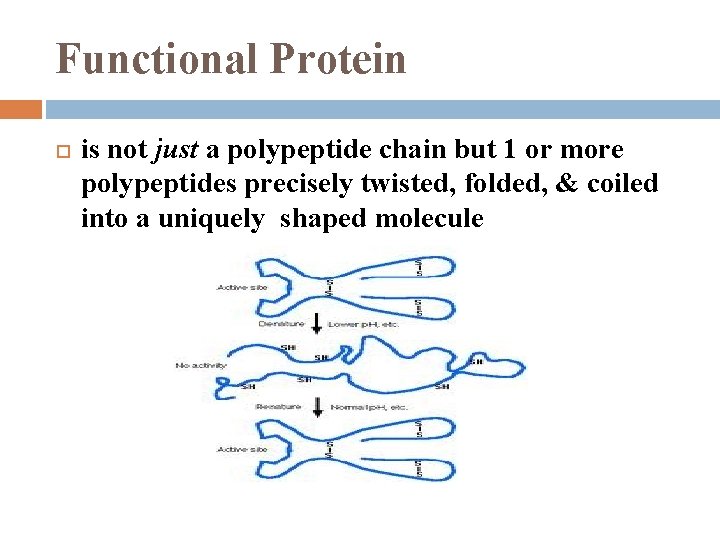
Functional Protein is not just a polypeptide chain but 1 or more polypeptides precisely twisted, folded, & coiled into a uniquely shaped molecule
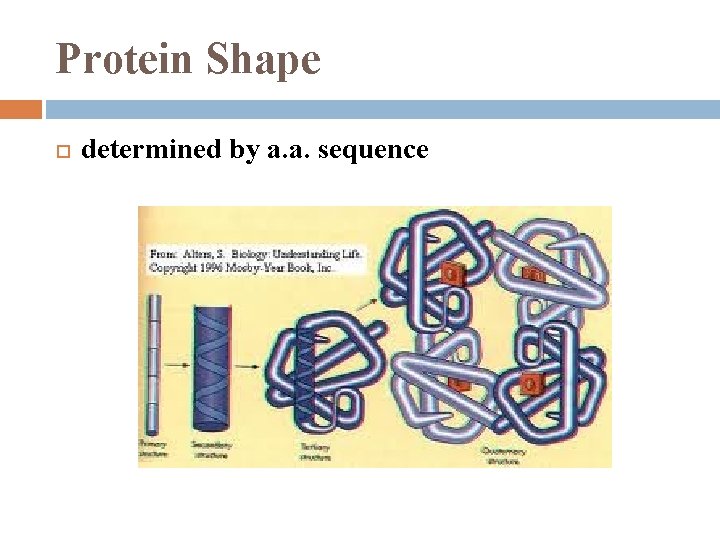
Protein Shape determined by a. a. sequence
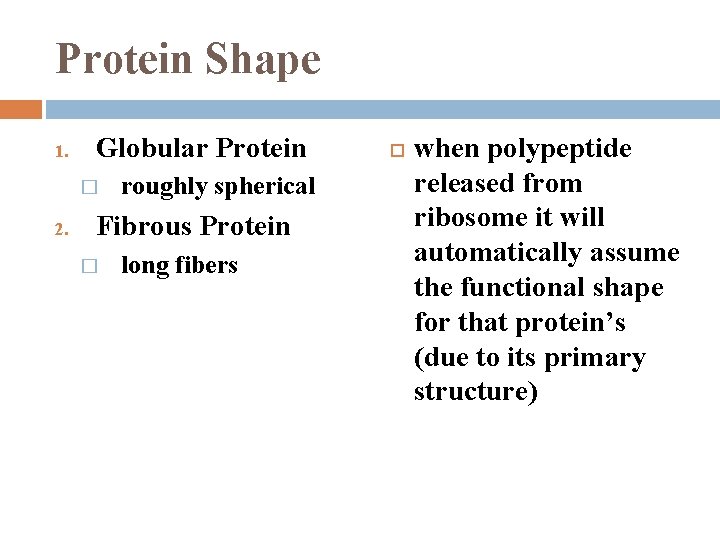
Protein Shape 1. Globular Protein � 2. roughly spherical Fibrous Protein � long fibers when polypeptide released from ribosome it will automatically assume the functional shape for that protein’s (due to its primary structure)
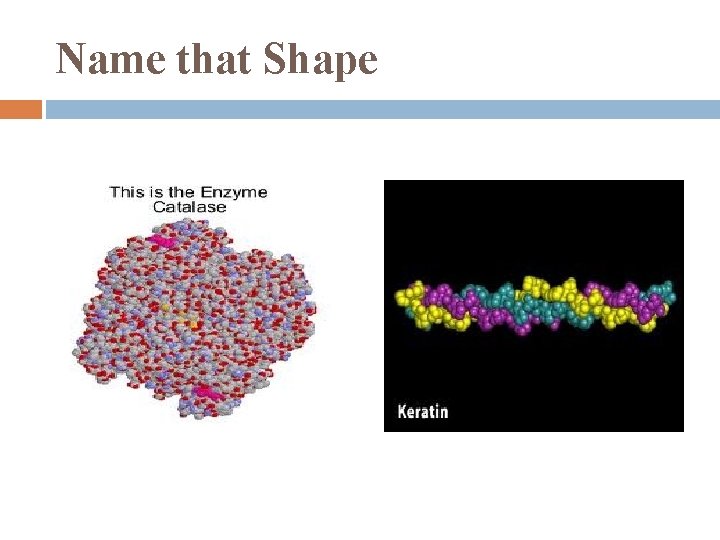
Name that Shape
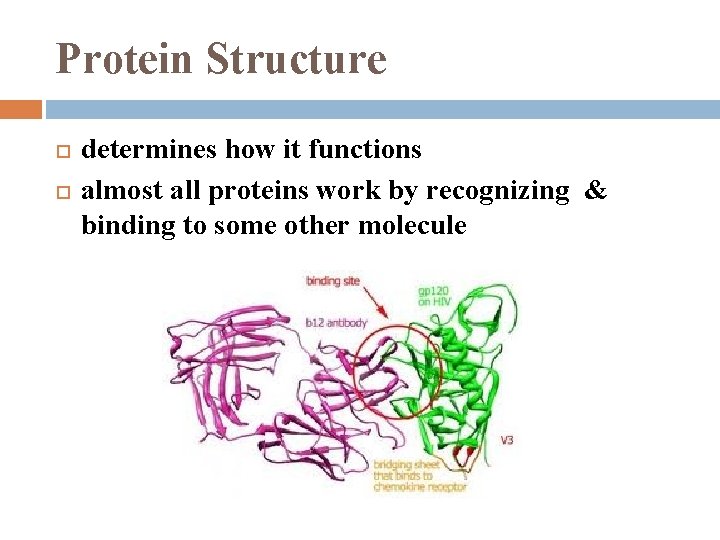
Protein Structure determines how it functions almost all proteins work by recognizing & binding to some other molecule
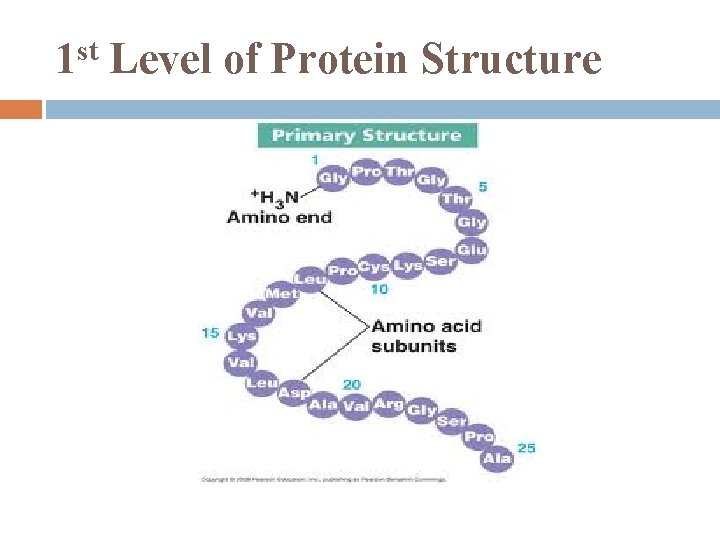
st 1 Level of Protein Structure
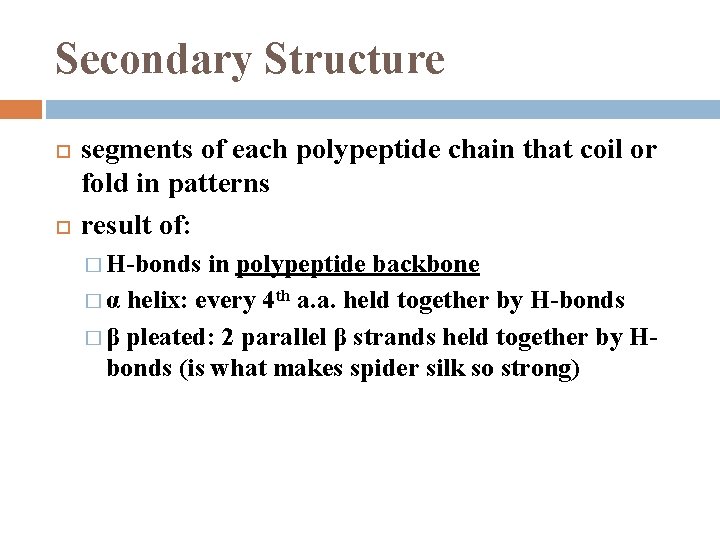
Secondary Structure segments of each polypeptide chain that coil or fold in patterns result of: � H-bonds in polypeptide backbone � α helix: every 4 th a. a. held together by H-bonds � β pleated: 2 parallel β strands held together by Hbonds (is what makes spider silk so strong)
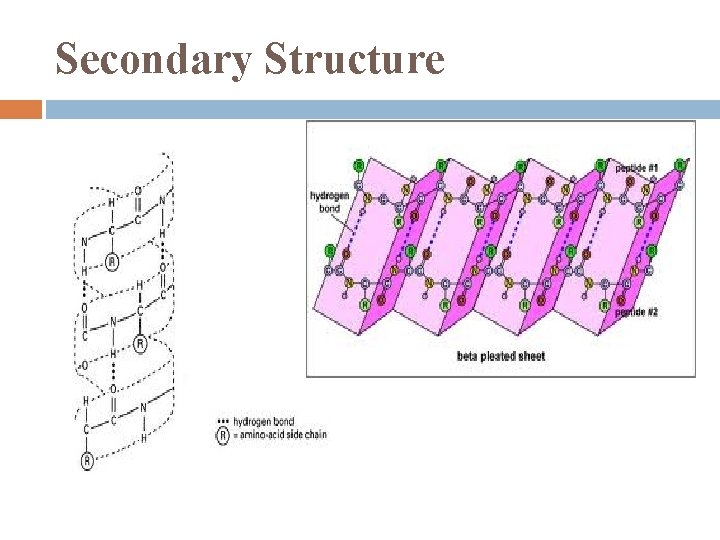
Secondary Structure
Tertiary Structure 1. 3 -D shape stabilized by interactions between side -chains hydrophobic interactions � � a. a. with nonpolar side chains usually end up together at core of protein: result of exclusion of nonpolar parts by water once nonpolar side chains away from water, van der Waals forces hold them together
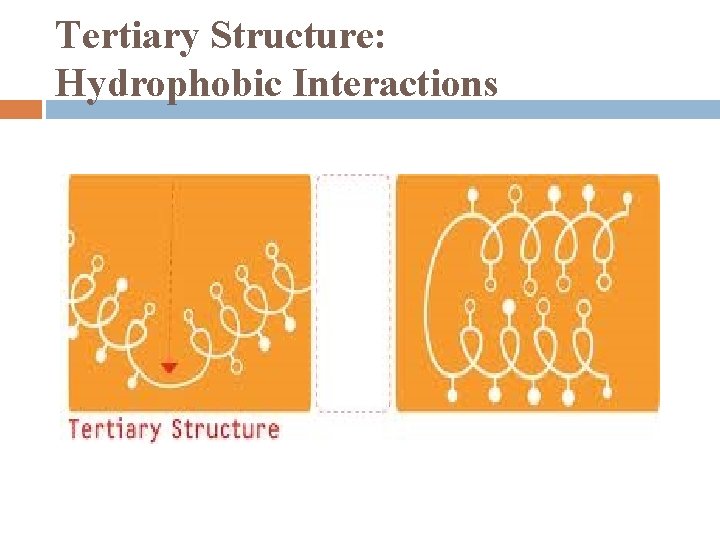
Tertiary Structure: Hydrophobic Interactions
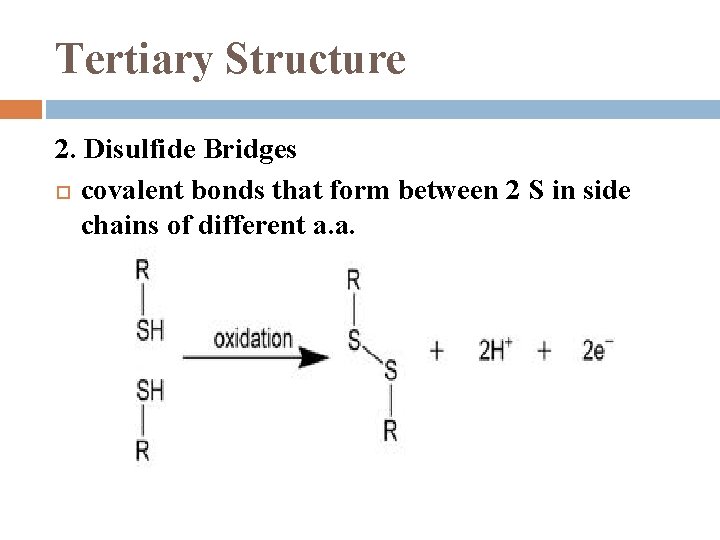
Tertiary Structure 2. Disulfide Bridges covalent bonds that form between 2 S in side chains of different a. a.
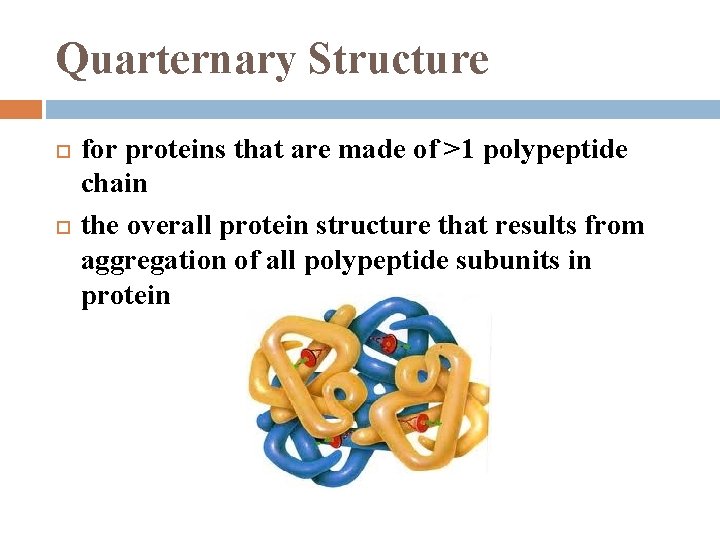
Quarternary Structure for proteins that are made of >1 polypeptide chain the overall protein structure that results from aggregation of all polypeptide subunits in protein
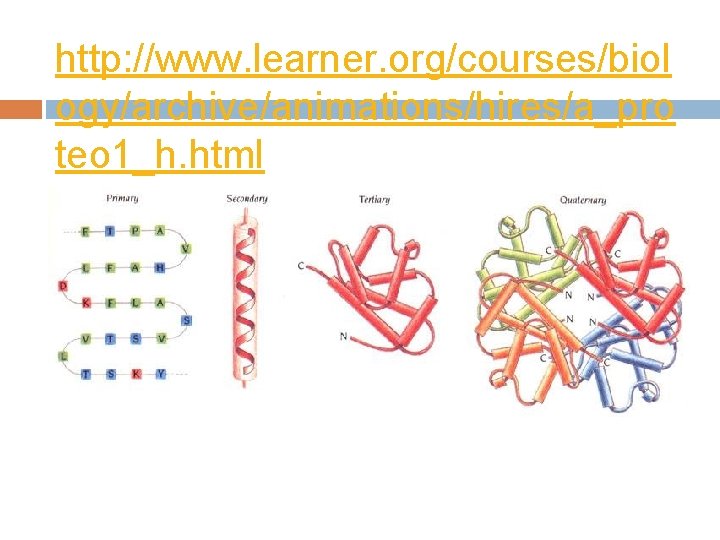
http: //www. learner. org/courses/biol ogy/archive/animations/hires/a_pro teo 1_h. html

Protein Structure http: //www. stolaf. edu/people/giannini/flashan imat/proteins/protein%20 structure. swf
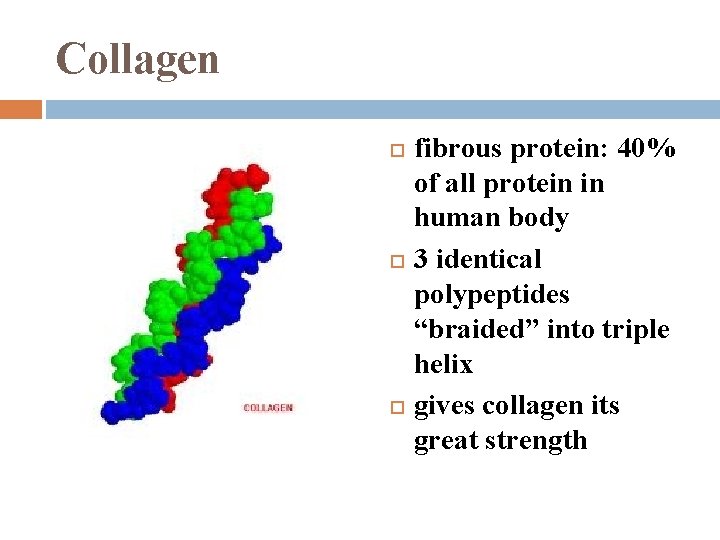
Collagen fibrous protein: 40% of all protein in human body 3 identical polypeptides “braided” into triple helix gives collagen its great strength
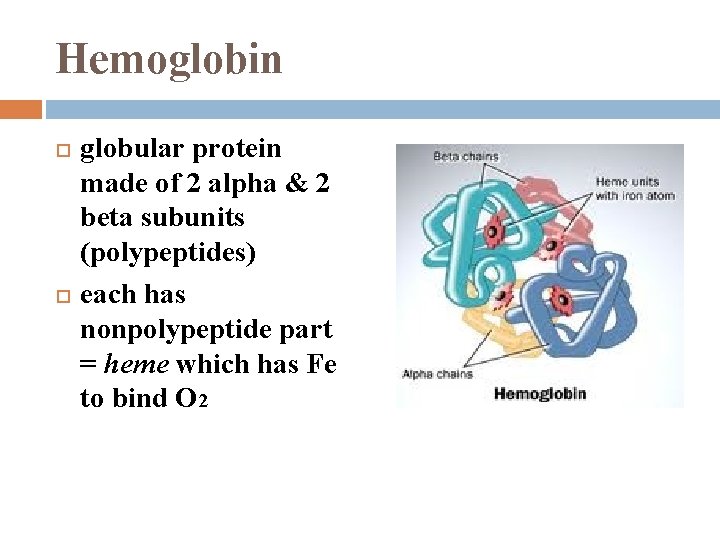
Hemoglobin globular protein made of 2 alpha & 2 beta subunits (polypeptides) each has nonpolypeptide part = heme which has Fe to bind O 2
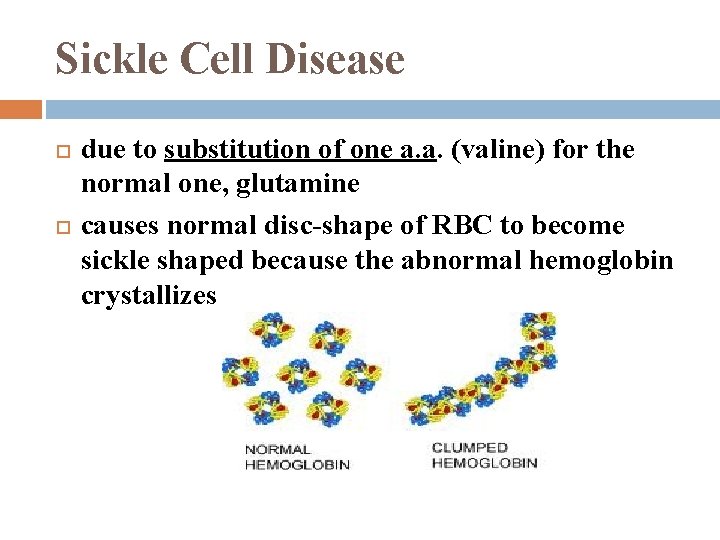
Sickle Cell Disease due to substitution of one a. a. (valine) for the normal one, glutamine causes normal disc-shape of RBC to become sickle shaped because the abnormal hemoglobin crystallizes
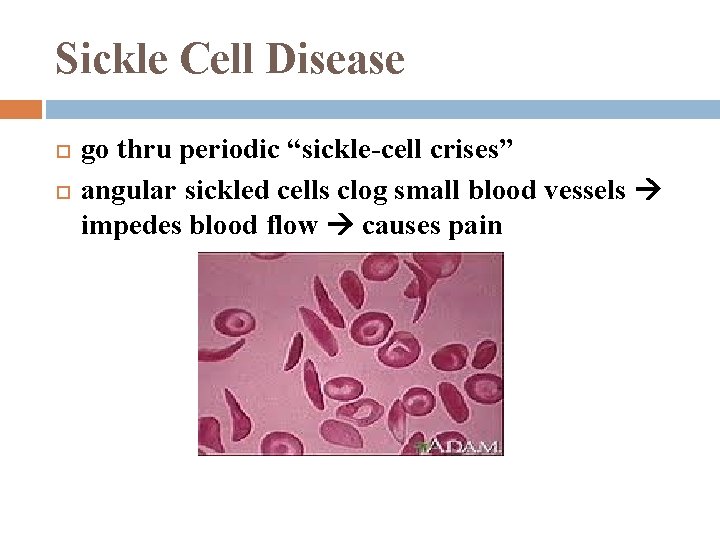
Sickle Cell Disease go thru periodic “sickle-cell crises” angular sickled cells clog small blood vessels impedes blood flow causes pain
Protein Structure 1. 2. 3. also depends on physical & chemical environment protein is in: p. H salt concentration temperature all of the above can change weak bonds & forces holding protein together
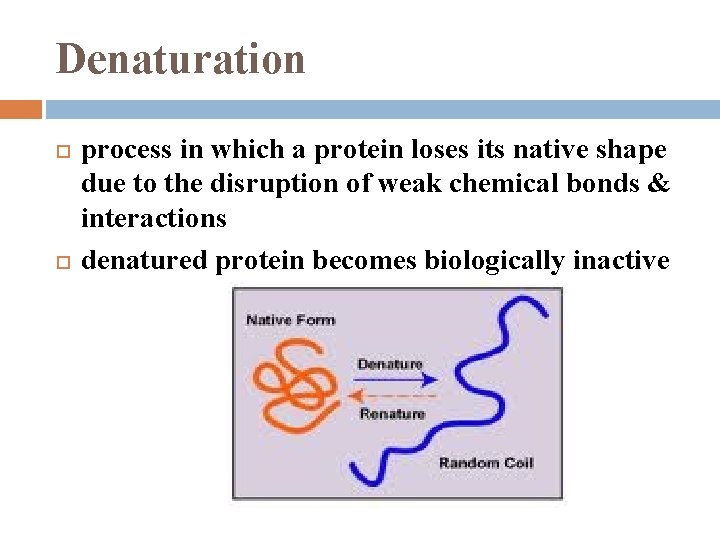
Denaturation process in which a protein loses its native shape due to the disruption of weak chemical bonds & interactions denatured protein becomes biologically inactive
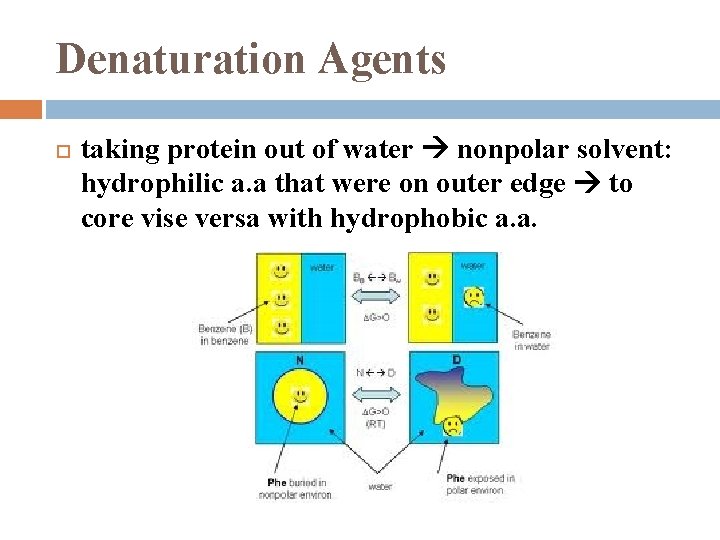
Denaturation Agents taking protein out of water nonpolar solvent: hydrophilic a. a that were on outer edge to core vise versa with hydrophobic a. a.
Protein Structure most proteins probably go thru some intermediate shape stages b/4 achieving their stable shape chaperonins: protein molecules that assist in the proper folding of other proteins
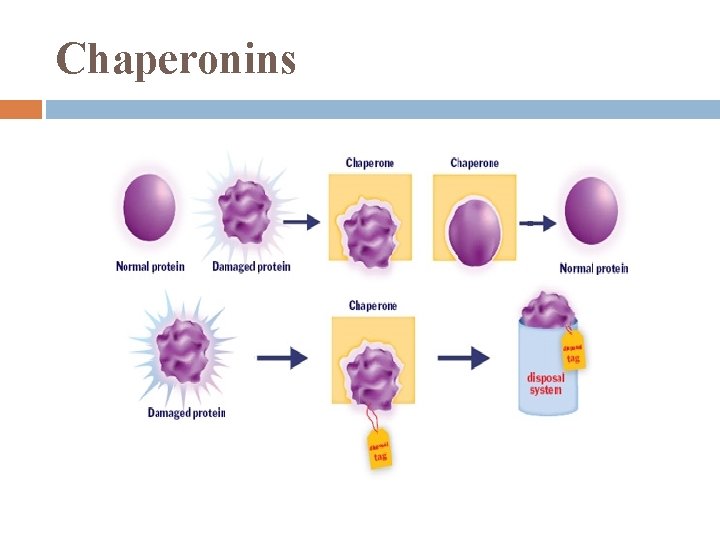
Chaperonins
Misfolded Proteins ass‘c with: � Alzheimer’s � Mad Cow disease � Parkinson’s � Senile Dementia
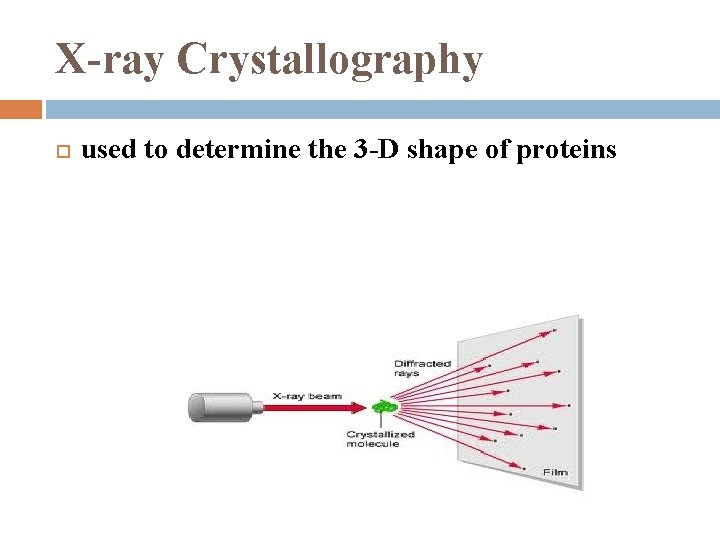
X-ray Crystallography used to determine the 3 -D shape of proteins
Nuclear Magnetic Resonance (NMR) Spectroscopy does not require crystallization of protein
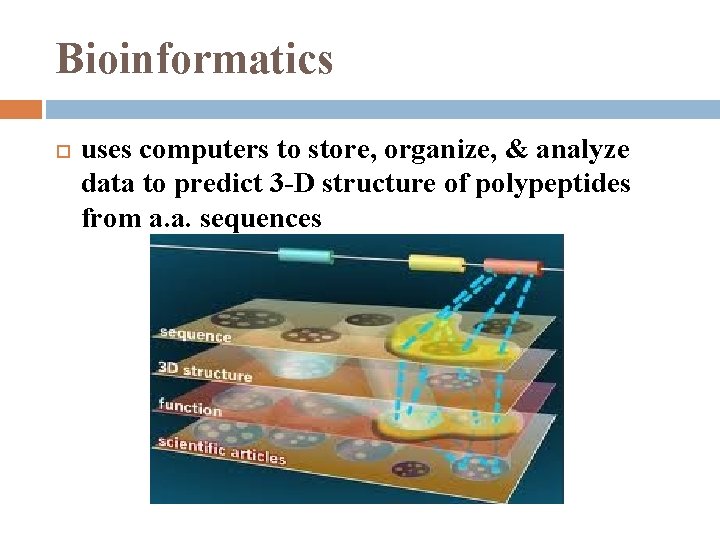
Bioinformatics uses computers to store, organize, & analyze data to predict 3 -D structure of polypeptides from a. a. sequences

NUCLEIC ACIDS 1. are polymers made of monomers called nucleotides genes code for a. a. sequences in proteins DNA deoxyribonucleic acid RNA ribonucleic acid
Nucleic Acid Roles DNA: 1. self-replication 2. reproduction of organism 3. flow of genetic information: DNA RNA synthesis protein synthesis
Nucleic Acid Roles RNA: 1. m. RNA � � � conveys genetic instructions for building proteins from DNA ribosomes in eukaryotic cells means from nucleus cytoplasm prokaryotic cells also use m. RNA
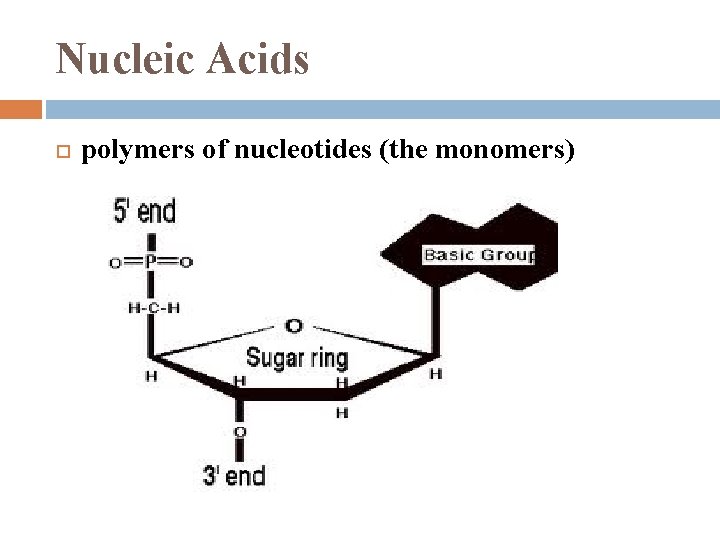
Nucleic Acids polymers of nucleotides (the monomers)
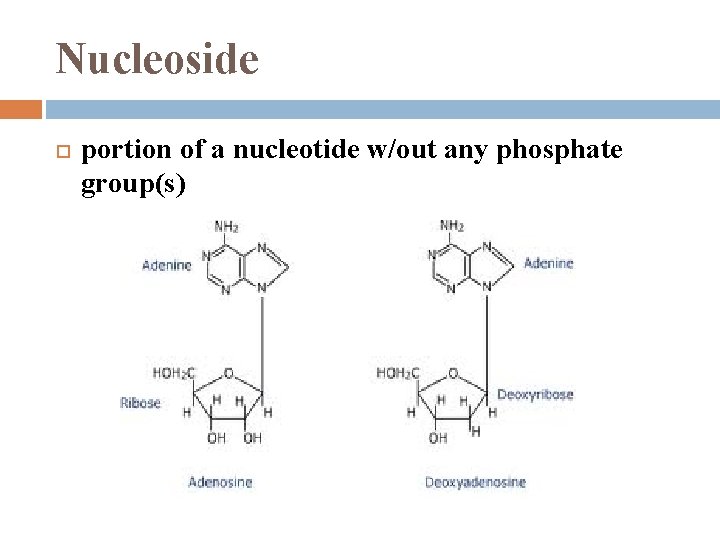
Nucleoside portion of a nucleotide w/out any phosphate group(s)

Nitrogenous Bases 1. each has 1 or 2 rings that include N are bases because the N atoms can take up H+ 2 families: Pyrimidines � 2. (1) 6 -sided ring made of C & N Purines � (1) 6 -sided ring fused to a 5 -sided ring
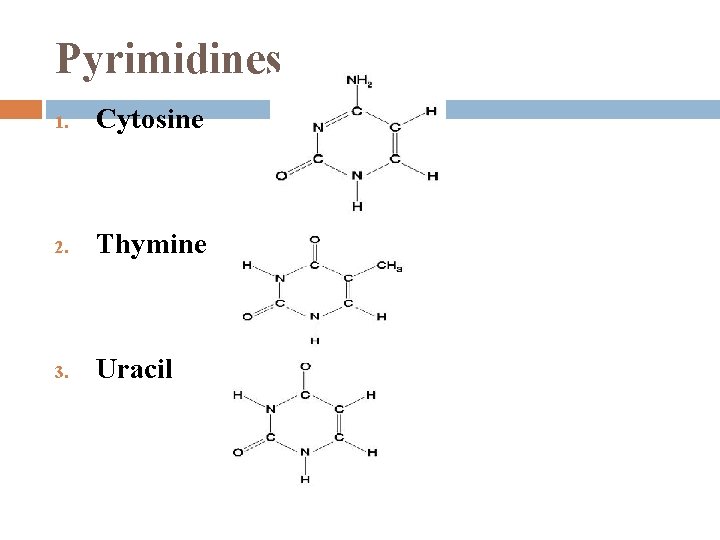
Pyrimidines 1. Cytosine 2. Thymine 3. Uracil
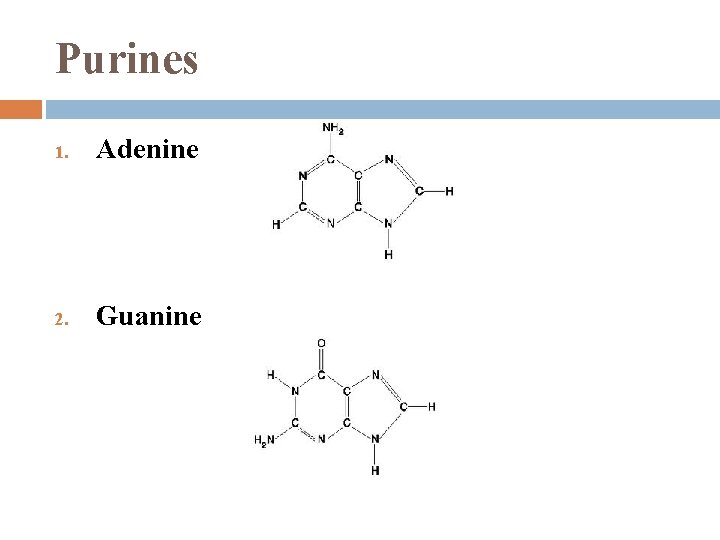
Purines 1. Adenine 2. Guanine
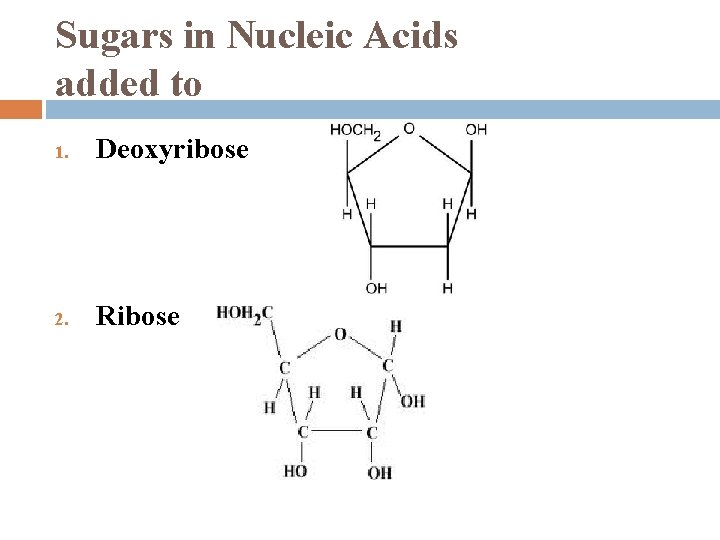
Sugars in Nucleic Acids added to 1. Deoxyribose 2. Ribose
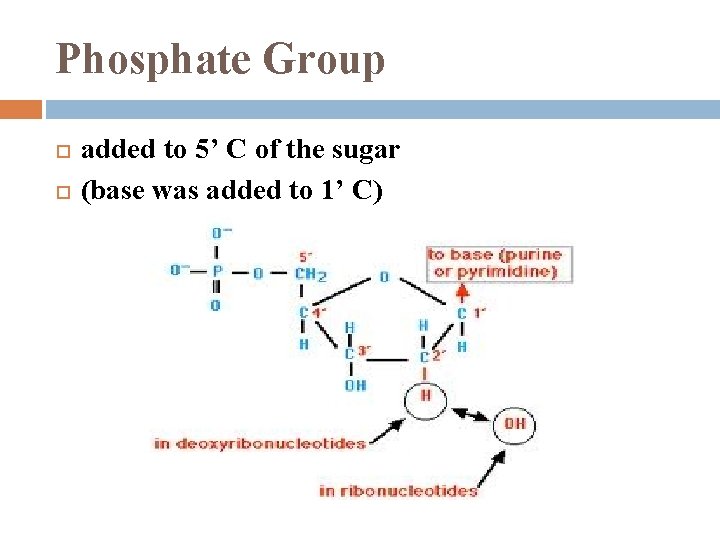
Phosphate Group added to 5’ C of the sugar (base was added to 1’ C)
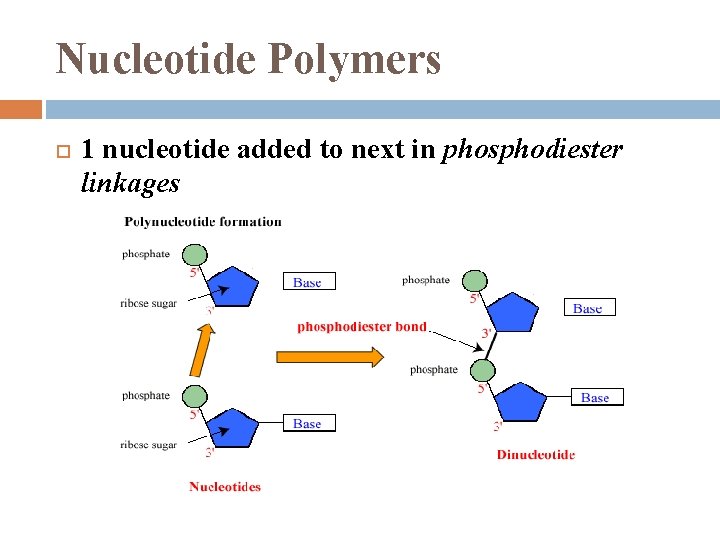
Nucleotide Polymers 1 nucleotide added to next in phosphodiester linkages
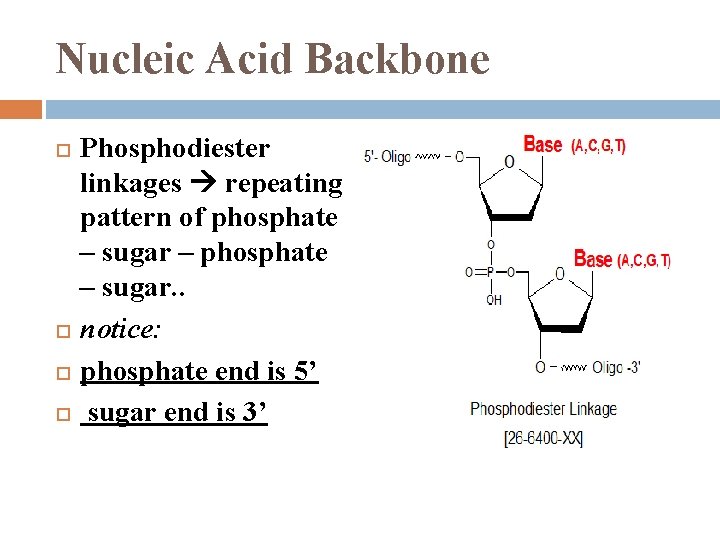
Nucleic Acid Backbone Phosphodiester linkages repeating pattern of phosphate – sugar – phosphate – sugar. . notice: phosphate end is 5’ sugar end is 3’
Polynucleotides have built-in direction along their sugar-phosphate backbones
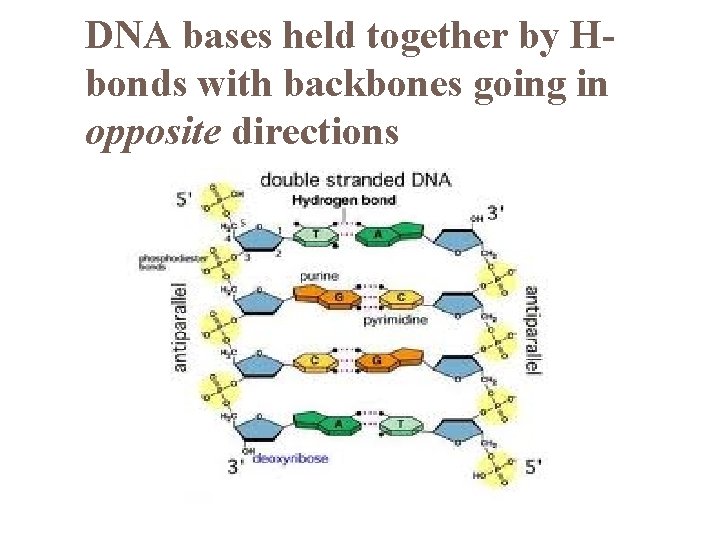
DNA bases held together by Hbonds with backbones going in opposite directions
Linear Order of Bases specifies start, stop of transcription/translation and codons determine primary structure of proteins (which determines the 3 -D structure of a protein which in turn determines the function of the protein)
DNA Molecules dbl stranded (in opposite directions = antiparallel) bases held together by H-Bonds most have thousands – millions base pairs bases pair using complementary base rules
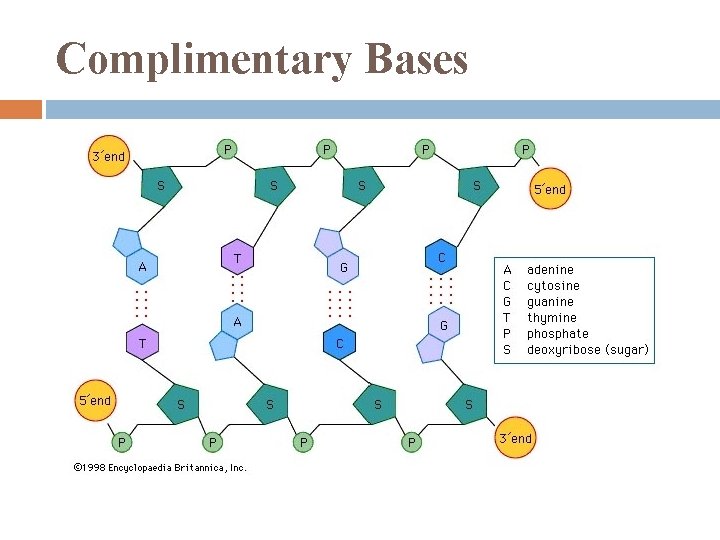
Complimentary Bases
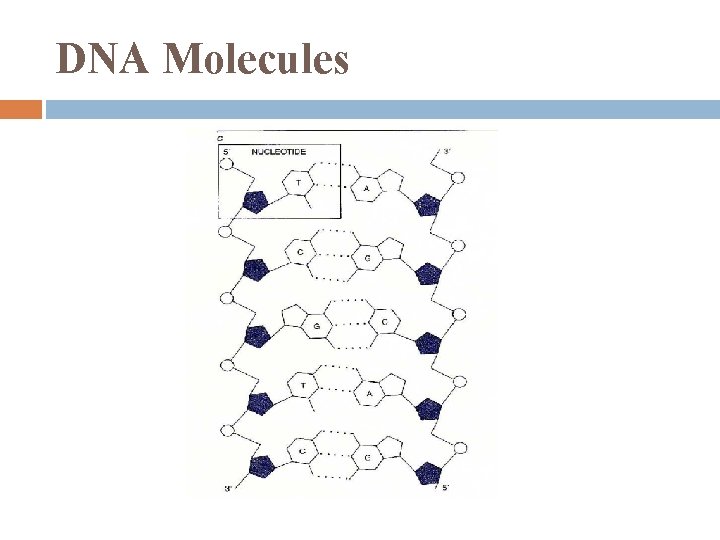
DNA Molecules
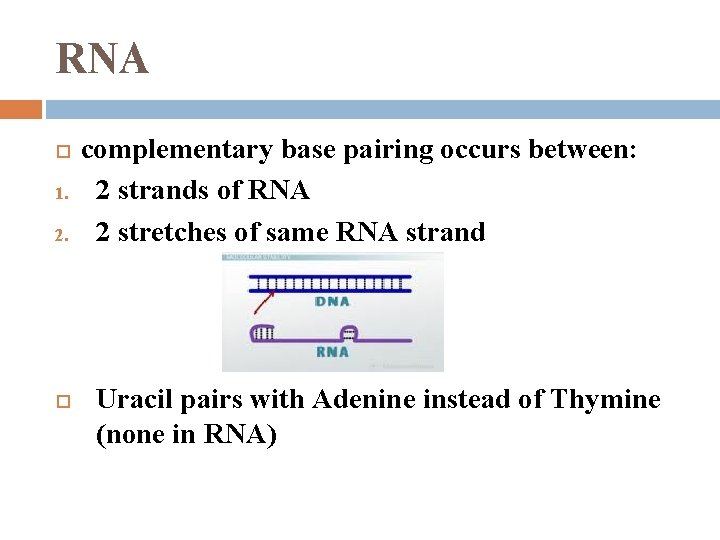
RNA 1. 2. complementary base pairing occurs between: 2 strands of RNA 2 stretches of same RNA strand Uracil pairs with Adenine instead of Thymine (none in RNA)
DNA & Proteins genes & their proteins document the hereditary background of an organism able to expect 2 species that appear to be closely related based on fossil & anatomical evidence to also share a greater proportion of their DNA & protein sequences than do more distantly related species

Hemoglobin human & gorilla hgb differ only by 1 a. a out of 46 in β chain human & frog differ by 67 a. a.
- Slides: 115