The Structure and Function of Macromolecules Campbell Chapter
The Structure and Function of Macromolecules Campbell Chapter 5
What is a macromolecule? • Macromolecules are large molecules that are composed of smaller units called monomers. The large molecule is known as a polymer. •
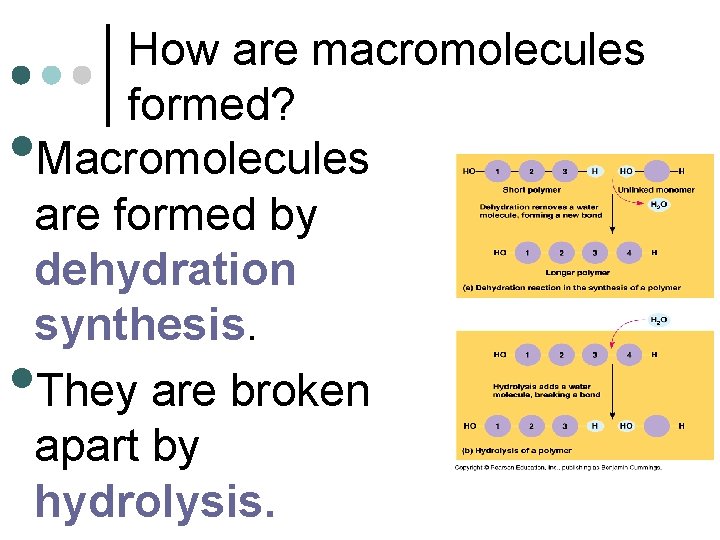
How are macromolecules formed? Macromolecules are formed by dehydration synthesis. They are broken apart by hydrolysis. • •

Today we’re going to talk about three major classes of macromolecules • Carbohydrates • Lipids • Proteins • The fourth, nucleic acids, will be discussed in greater detail later this semester.
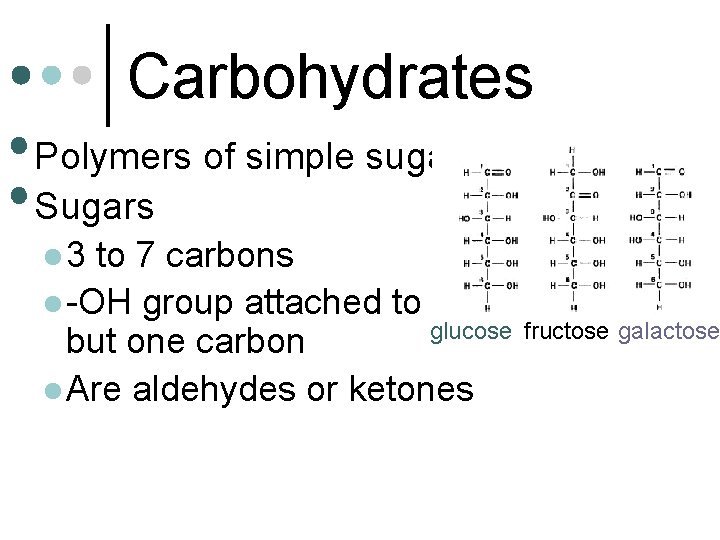
Carbohydrates • Polymers of simple sugars • Sugars l 3 to 7 carbons l -OH group attached to all glucose but one carbon l Are aldehydes or ketones fructose galactose
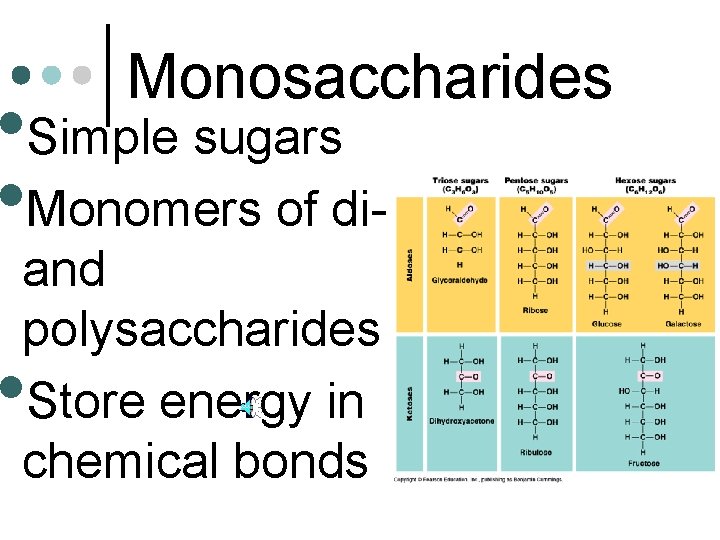
Monosaccharides • Simple sugars • Monomers of diand polysaccharides Store energy in chemical bonds •
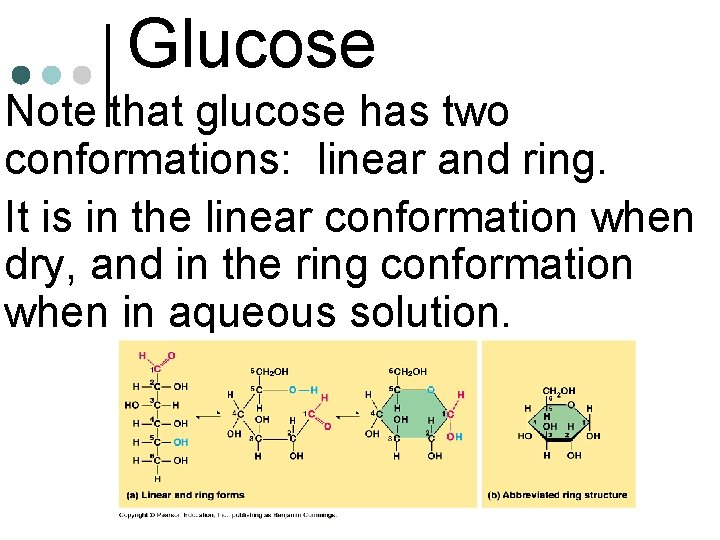
Glucose • Note that glucose has two conformations: linear and ring. • It is in the linear conformation when dry, and in the ring conformation when in aqueous solution.
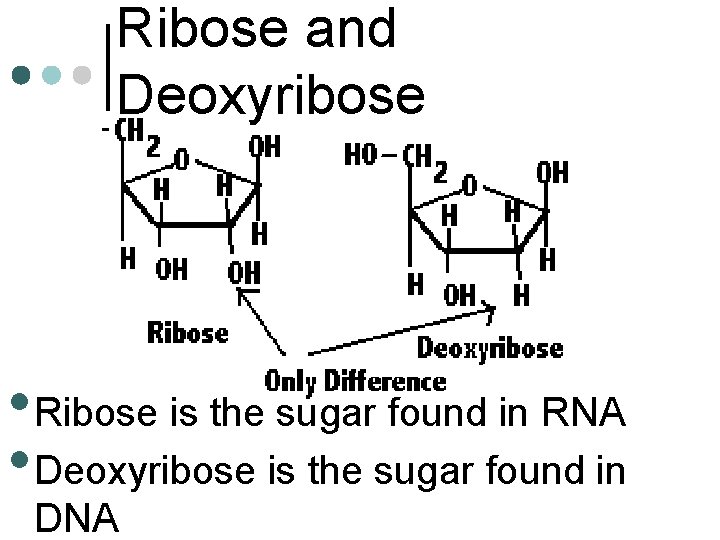
Ribose and Deoxyribose • Ribose is the sugar found in RNA • Deoxyribose is the sugar found in DNA

Disaccharides • “Double sugars” • Formed by dehydration synthesis • The removal of a water molecule • forms a glycosidic linkage between monomers Sucrose, maltose, lactose are examples
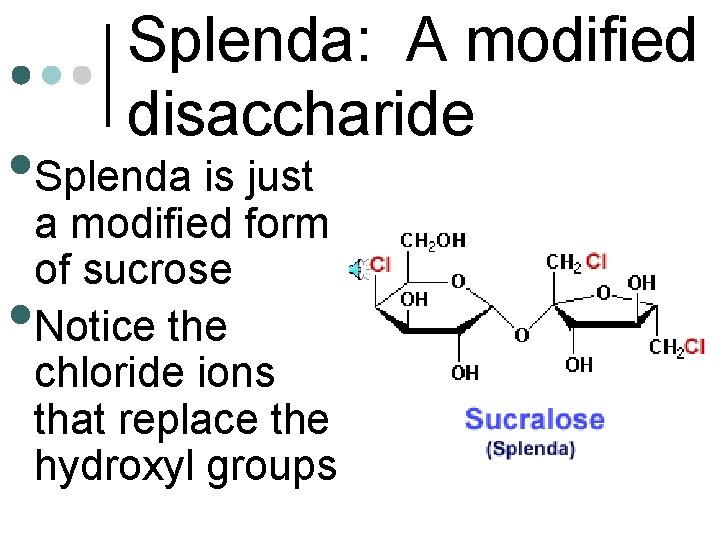
Splenda: A modified disaccharide • Splenda is just • a modified form of sucrose Notice the chloride ions that replace the hydroxyl groups
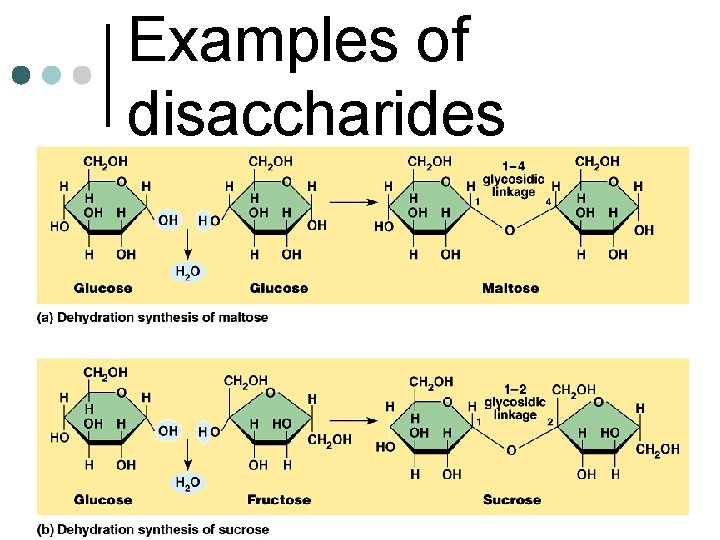
Examples of disaccharides
Polysaccharides • Many monosaccharides covalently bound together Function as storage molecules and structural molecules •

Storage polysaccharides • Starch: carb that plant tissues store Glycogen: carb that animal tissues store •
Structural polysaccharides • Cellulose: makes up plant cell walls Chitin: polymer of amino sugars. Makes up exoskeletons. •
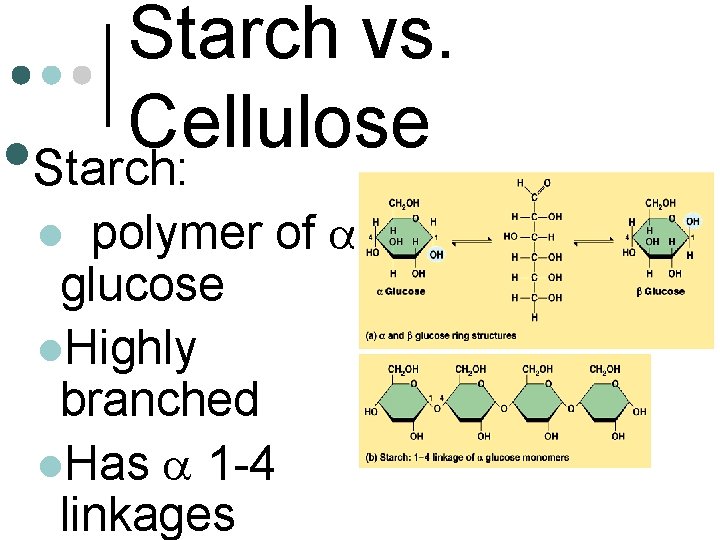
Starch vs. Cellulose • Starch: polymer of aglucose l. Highly branched l. Has a 1 -4 linkages l
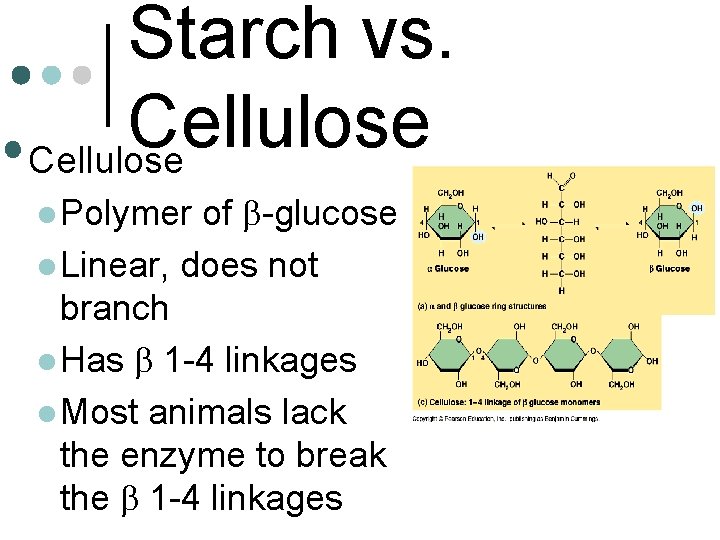
Starch vs. Cellulose • Cellulose of b-glucose l Linear, does not branch l Has b 1 -4 linkages l Most animals lack the enzyme to break the b 1 -4 linkages l Polymer

Lipids • Insoluble in water • Mostly long hydrocarbon chains Fats, steroids, phospholipids •
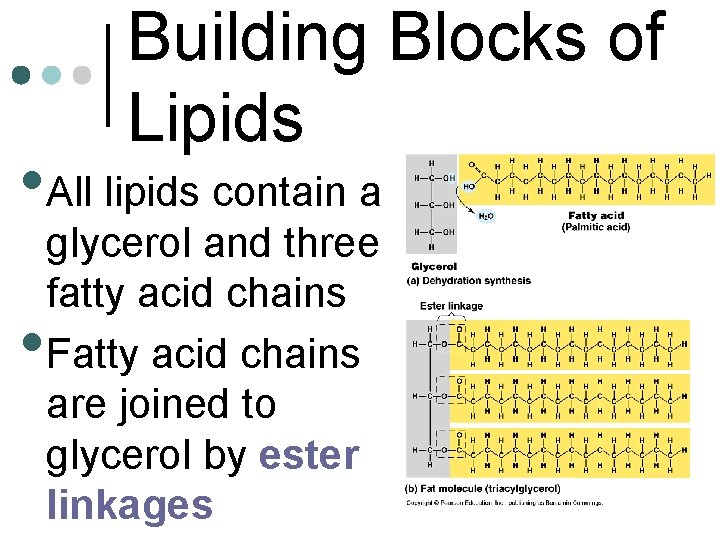
Building Blocks of Lipids • All lipids contain a • glycerol and three fatty acid chains Fatty acid chains are joined to glycerol by ester linkages
Fats • Glycerol + fatty acids • Triglycerides have 3 fatty acids which can vary • Act as compact energy source • Insulates and cushions vital organs • Two types: saturated and unsaturated
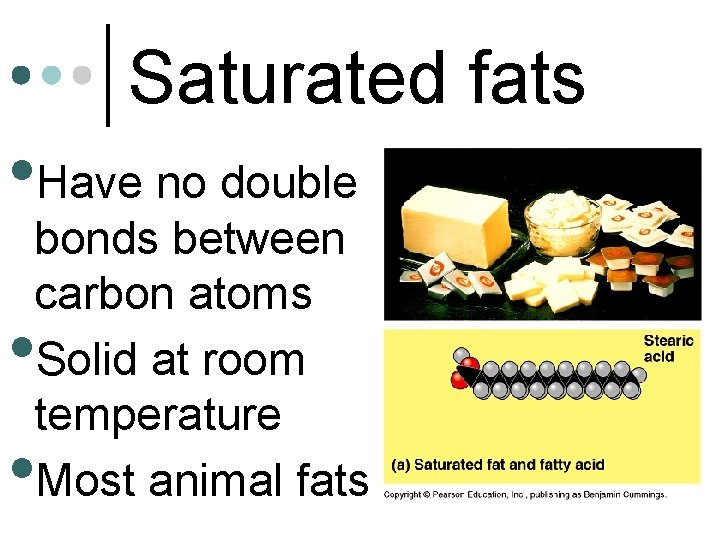
Saturated fats • Have no double bonds between carbon atoms Solid at room temperature Most animal fats • •

Unsaturated fats • At least 1 double • • • bond between carbons Hydrocarbon chain is bent Usually liquid at room temp Most plant fats
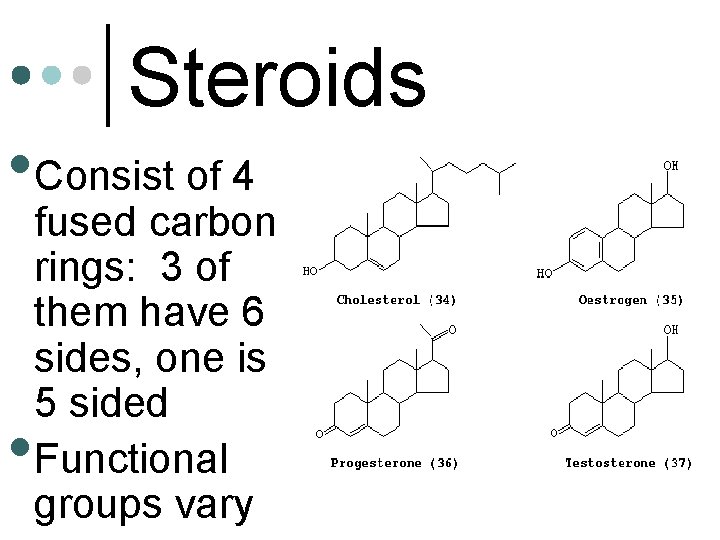
Steroids • Consist of 4 • fused carbon rings: 3 of them have 6 sides, one is 5 sided Functional groups vary
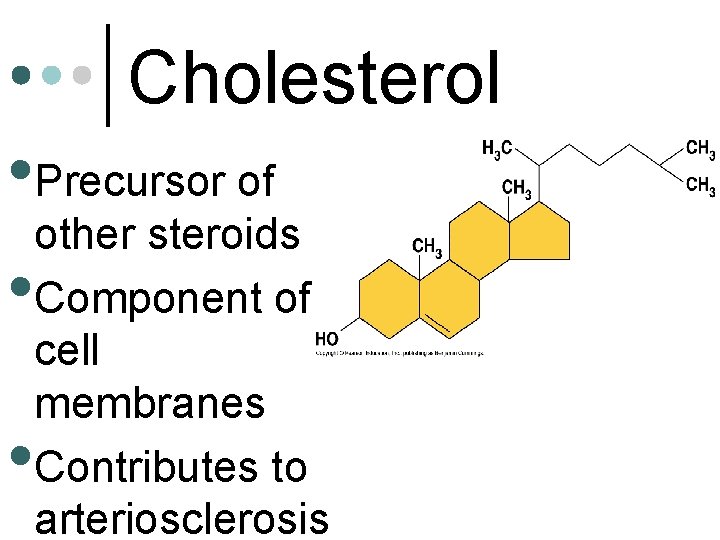
Cholesterol • Precursor of other steroids • Component of cell membranes Contributes to arteriosclerosis •
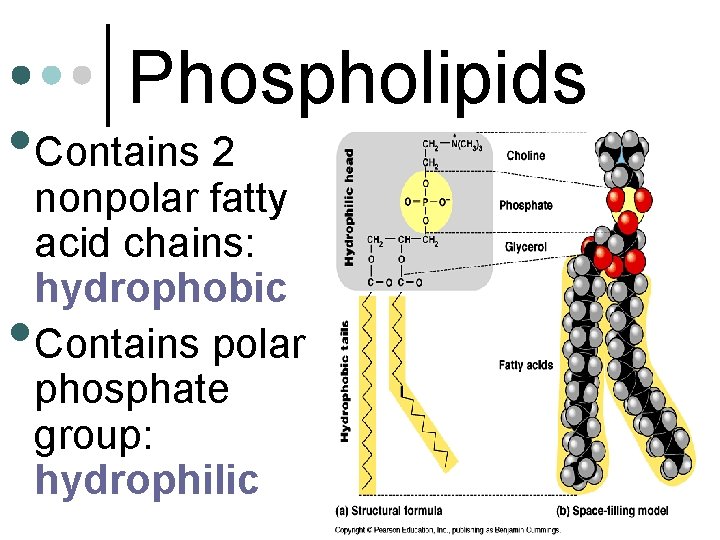
Phospholipids • Contains 2 • nonpolar fatty acid chains: hydrophobic Contains polar phosphate group: hydrophilic
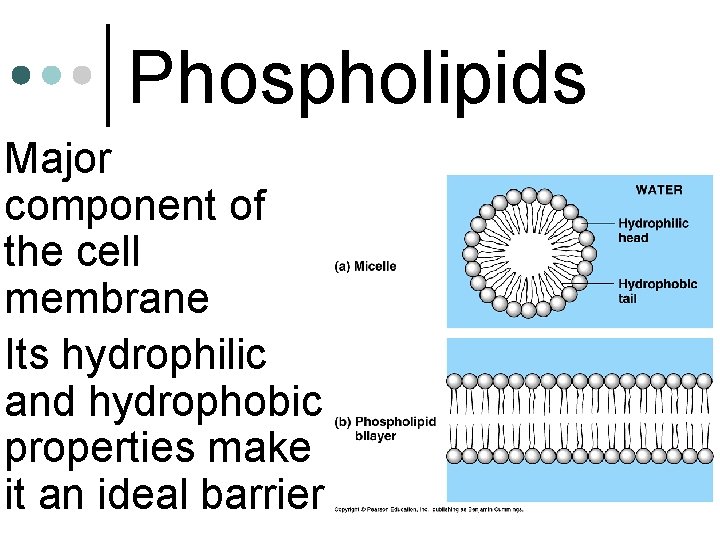
• Major • Phospholipids component of the cell membrane Its hydrophilic and hydrophobic properties make it an ideal barrier
Proteins • Polymers of amino acids • Each has a unique 3 D shape • Amino acid sequences vary • Major component of cell parts • Provide support and structure • Storage of amino acids • Several types: receptor, contractile, defense, enzymes, structural
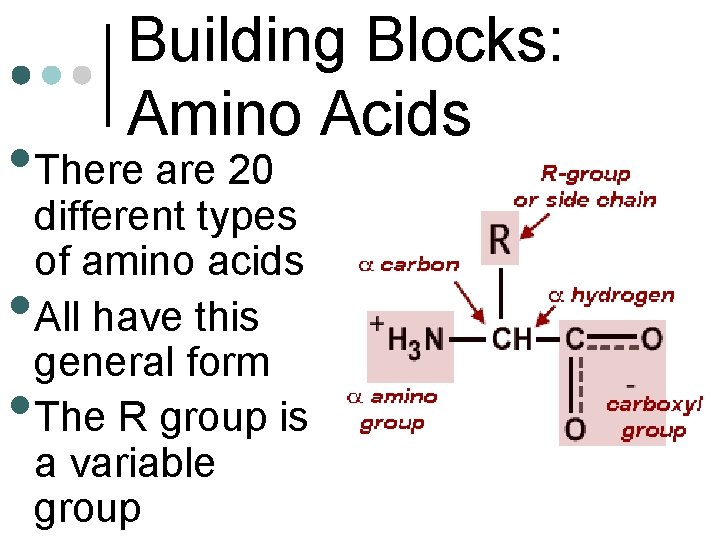
Building Blocks: Amino Acids • There are 20 • • different types of amino acids All have this general form The R group is a variable group
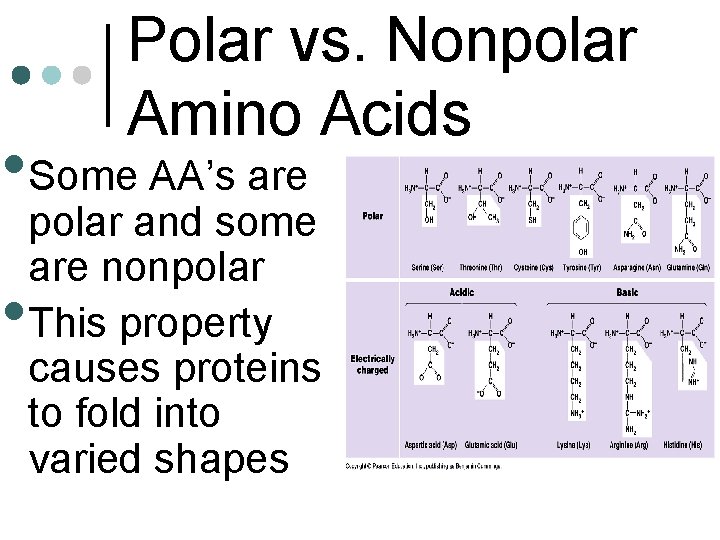
Polar vs. Nonpolar Amino Acids • Some AA’s are • polar and some are nonpolar This property causes proteins to fold into varied shapes
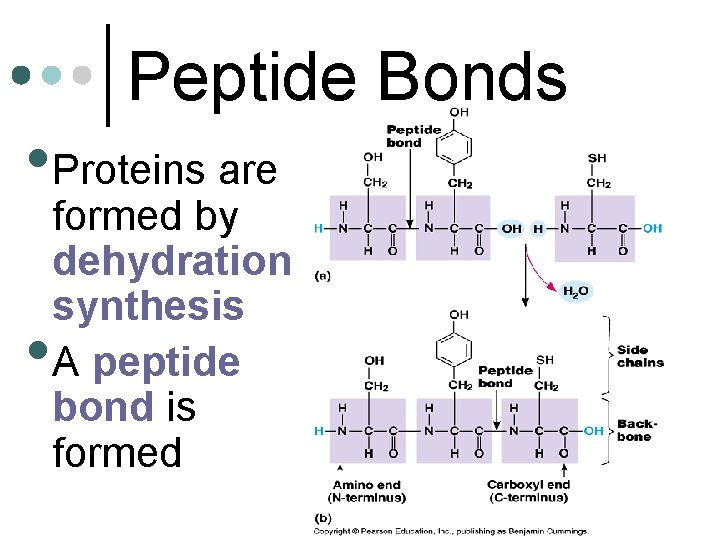
Peptide Bonds • Proteins are • formed by dehydration synthesis A peptide bond is formed
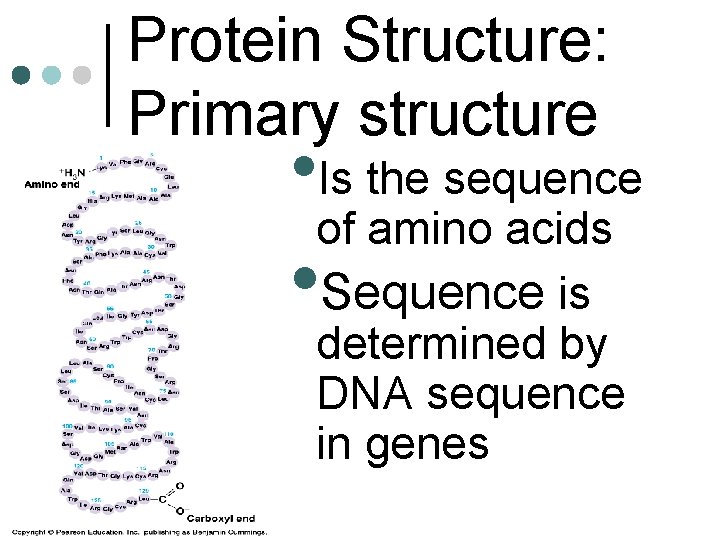
Protein Structure: Primary structure • Is the sequence of amino acids Sequence is determined by DNA sequence in genes •
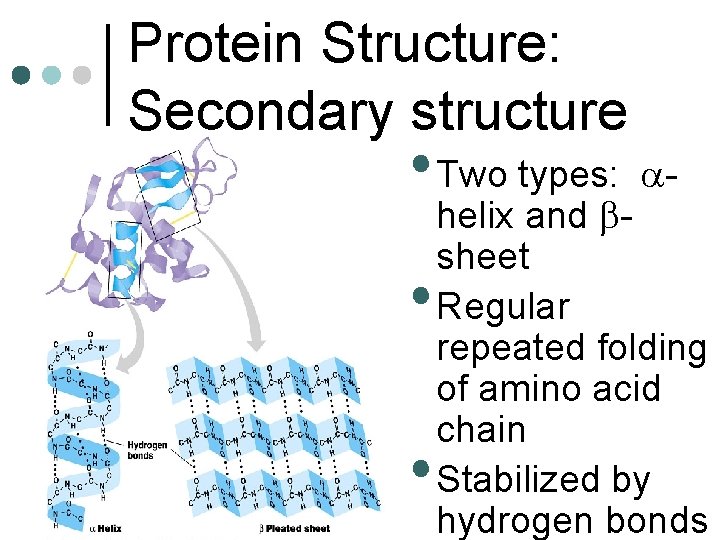
Protein Structure: Secondary structure • Two types: • • a- helix and bsheet Regular repeated folding of amino acid chain Stabilized by hydrogen bonds
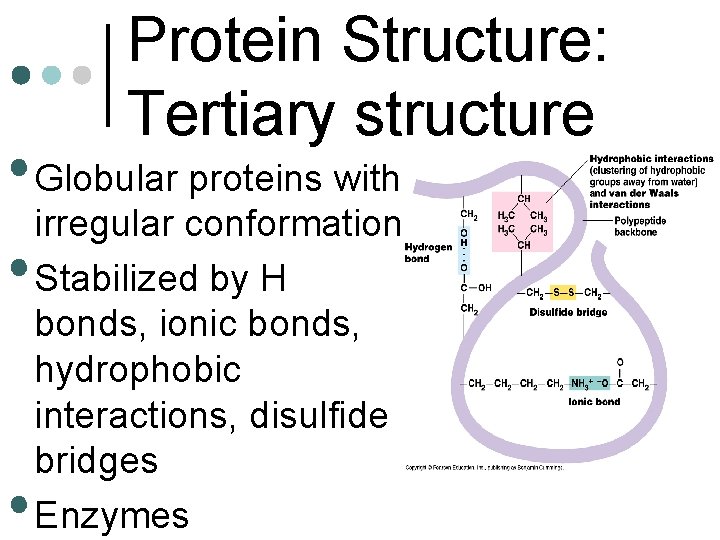
Protein Structure: Tertiary structure • Globular proteins with irregular conformation • Stabilized by H • bonds, ionic bonds, hydrophobic interactions, disulfide bridges Enzymes
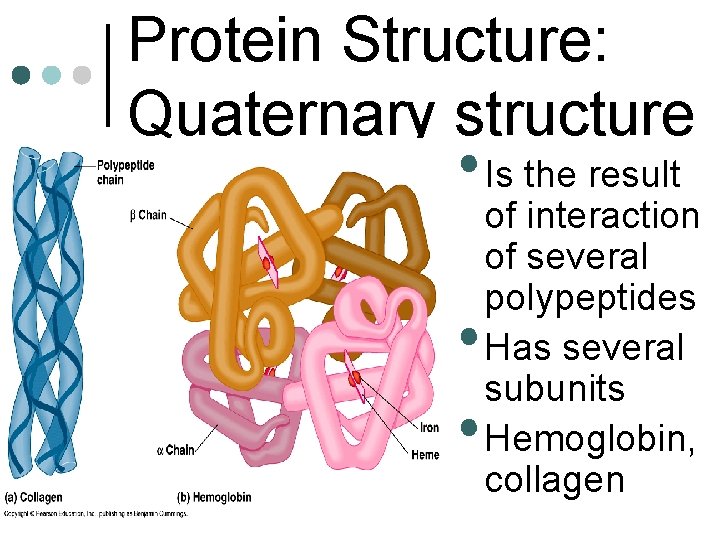
Protein Structure: Quaternary structure • Is the result • • of interaction of several polypeptides Has several subunits Hemoglobin, collagen
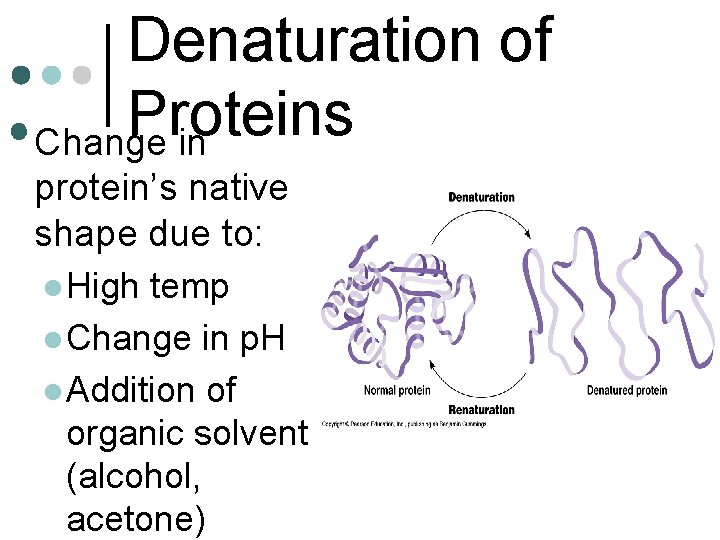
Denaturation of Proteins • Change in protein’s native shape due to: l High temp l Change in p. H l Addition of organic solvent (alcohol, acetone)
Nucleic Acids • Store and transmit genetic information Two types: DNA, RNA Made of nucleotides • •
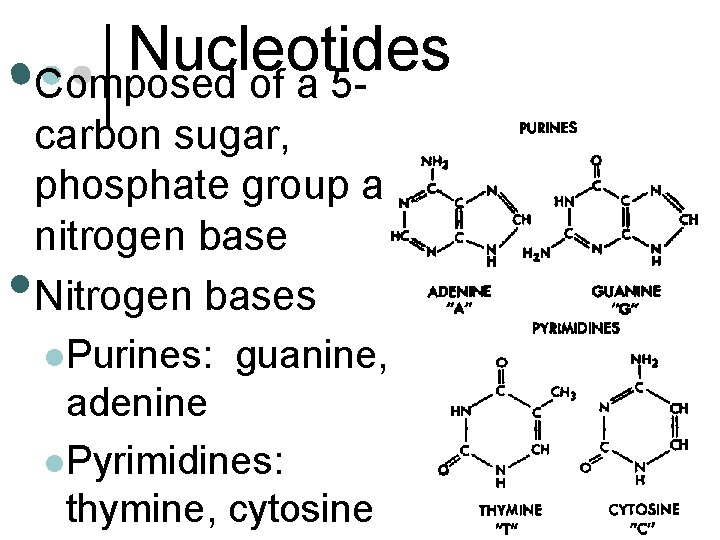
Nucleotides • Composed of a 5 - • carbon sugar, phosphate group and nitrogen base Nitrogen bases l. Purines: guanine, adenine l. Pyrimidines: thymine, cytosine
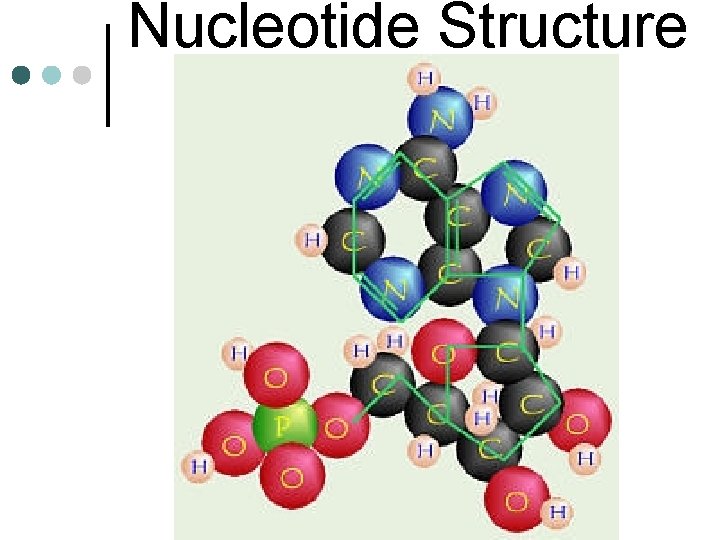
Nucleotide Structure
- Slides: 37