The SternGerlach Experiment and the nonDiscovery of the















































- Slides: 53

The Stern-Gerlach Experiment and the non-Discovery(? ) of the Electron Spin Sandip Pakvasa University of Hawaii Honolulu Hawaii Nov. 7, 2017. NTU, Taipei

In 1990, at the Neutrino ‘ 90 conference, Leon Lederman complained that theorists are always lionized, whereas experimentalists are very often short-changed and not given enough credit! As an example, he cited Pauli getting a lot of credit for the invention of the neutrino in 1930, but the very hard work of Charles Drummond Ellis and his collaborators as well as Lise Meitner who labored very hard from 1920 to 1929 to firmly establish that the beta decay spectrum is truly continuous , which made Pauli’s hypothesis necessary , were hardly acknowledged by the community…. ! I try to redress this iniquity……in my talks. Now onto Stern-Gerlach….

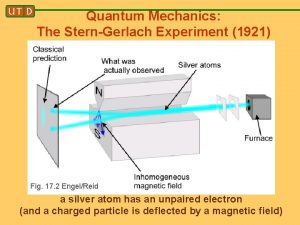
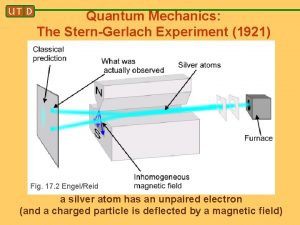
In many textbooks, we learn about the Stern-Gerlach experiment as a classic way to measure spin and illustrate fundamental quantum mechanical behaviour e. g. entanglement etc. and why it is so important in the discussion of the measurement problem usually early in the first chapter e. g. Bohm(1952)(pioneer) Merzbacher(1962) Feynman(1965) Messiah(1966 ) Gottfried(1966) Baym(1969) Sakurai(1985) Abers(2004) Weinberg(2013) etc

We also learn that the Stern-Gerlach experiment Was performed and published in 1921 -2! A few pages later, the same textbooks ascribe the “Discovery” of electron spin to theoretical proposal by Goudsmit and Uhlenbeck in 1925! How come the discovery of electron spin was not ascribed to Stern and Gerlach? And due credit not given to them? I started worrying about this anomaly only after having been teaching Quantum Mechanics for over 40 years and never having questioned it(well, almost never)! In the process I learnt , among other things, a lot about the original experiment, the troubles with its interpretation, the astoundingly creative career of Otto Stern and other tidbits (with crucial help from my colleague Xerxes Tata)

Otto Stern was born in Sohrau, Germany in 1888, and received his Ph. D. in 1912, from Breslau Univ. He joined Einstein in Prague as his first “post-doc”, and followed Einstein to Zurich. (He became a lifelong friend of Einstein and visited him in Princeton during 1950’s). In Zurich, he became close friend and companion of Max von Laue (and later many other theorists including Pauli) They shared profound misgivings about the atomic model of Bohr published in 1913. While hiking on a mountain near Zurich, they took what Pauli later called “Utlischwur”: ”If this nonsense of Bohr should prove to be right in the end, we will quit physics”! Needless to say, Bohr was right AND they did NOT quit physics!

This was a joke on the famous Swiss oath rutlischwur which refers to William Tell and the rebellion against the ruling Austrians, in 14 th century and this was an oath about freedom for the Swiss…….

He spent the war years(1914 -18) in the army and worked as a meteorologist on the eastern front. In Frankfurt, he carried out the measurement of velocities of molecules emitted from a heated wire and confirmed the Maxwell-Boltzmann distribution, in an ingenious experiment. The molecular beam technique was invented by L. Dunoyer(1911) but exploited thoroughly by Stern.

Stern ended up in Frankfurt with Max Born as his mentor. Finally, in early 1921, Stern thought he had come up with an idea to disprove the Bohr “orbits”! In the meantime the Bohr model had been embellished by Sommerfeld and others to include eliptical orbits, orbital angular momentum etc. And Debye and Sommerfeld had described the orbits with magnetic moments orientable in magnetic fields. Since orbits can only be in certain planes w. r. t. the magnetic field direction, this was called “space quantization”. Stern thought he could test this idea experimentally. In august 1921, he submitted a paper to ‘Zeitschrift fur physik’ entitled “ A way towards the experimental examination of spatial quantization in a magnetic field” Of course, he was hoping to prove it wrong!

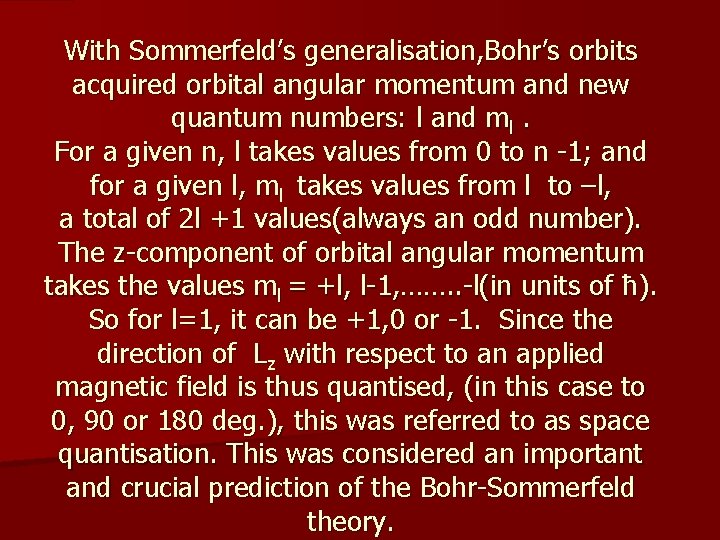
With Sommerfeld’s generalisation, Bohr’s orbits acquired orbital angular momentum and new quantum numbers: l and ml. For a given n, l takes values from 0 to n -1; and for a given l, ml takes values from l to –l, a total of 2 l +1 values(always an odd number). The z-component of orbital angular momentum takes the values ml = +l, l-1, ……. . -l(in units of ħ). So for l=1, it can be +1, 0 or -1. Since the direction of Lz with respect to an applied magnetic field is thus quantised, (in this case to 0, 90 or 180 deg. ), this was referred to as space quantisation. This was considered an important and crucial prediction of the Bohr-Sommerfeld theory.


Although in Debye-Sommerfeld proposal, for l=1, there should be three possible values of ml = +1, 0 and -1, and hence three orientations of the orbit; in 1918 in a series of papers on atomic spectra, Bohr said that he was troubled by the case ml=0, because it corresponds to the orbit being parallel to the magnetic field direction and is hence “unstable”! (essentially, ” I don’t like it!” kind of an argument). Sommerfeld in his influential textbook repeated Bohr’s claim. This is the reason why, when Stern wrote the paper with his proposed experiment, following this lead(from eminent theorists), he also said that they would see the beam split in two!

In the paper he already mentions that he and Gerlach have started to work on the proposed experiment. He had realised how difficult the experiment was and found a collaborator: Walter Gerlach who was already in Frankfurt, and a superb experimenter. By arly 1921, they had already started on the design and execution of the experiment experimen They were not encouraged by theorists, even sympathetic ones like Born! As Born recalled: ”In fact it took me quite a while before I took this idea seriouslyl! I thought that space quantisation was a kind of symbolic expression for something which you did not understand. But to take it literally like Stern did, this was his own idea. . I tried to persuade Stern that there was no sense to it, but then he told me that it was worth a try. ” Eventually Born came around and became an enthusiastic supporter of the experiment. Although, at one time when they did not see any effect, Debye said, ”surely you did not really believe that the orientation of orbits will be physically real…”.

A note on Stern’s style of working: He always had a cigar in one hand, and he left actual work with hands to others, as he did not trust his own manual dexterity! If there was an imminent crash, he would raise both his hands and stay away, as he said that it is better to let things fall where they may, rather than trying to prevent the fall! He described the beneficial effects of a large wooden hammer that he kept in his lab and used it to threaten the apparatus if it did not behave!(apparently it worked!)

The experiment turned out to be even more difficult than anticipated. It took more than a year to complete. There were also financial difficulties. A series of public lectures by Born and others were arranged with a charge for admission to help defray the cost of the experiment! A friend suggested to Born to write to a Henry Goldman(in New York) who had family roots in Frankfurt. He received a charming response with a cheque for a few hundred dollars , which helped them tide over…. (Henry Goldman had started the Woolworth chain and founded Goldman. Sachs. ) In the meantime, Born had moved to Gottingen and Stern had moved to Rostock. Gerlach had to travel to Rostock to show the results to Stern…On one such visit, they reviewed the results, and in view of disappointing results, decided to quit! However, Gerlach had to spend the night in Rostock due to a railway strike, and decided to try one more time when he got back to Frankfurt! And this time they had success!

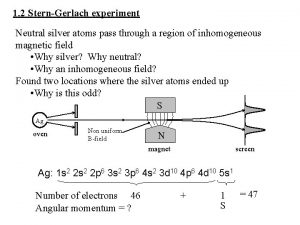
W. Gerlach and O. Stern, Z. f. Physik. 9, 353(1922): actual values: L=3. 5 cm, B=0. 1 T, gradient of 10 T/cm, resultant Δz = 0. 22 mm. They saw two lines, and the value of Δz corresponds to a value of magnetic moment of exactly one Bohr magneton as expected! The shift was larger than Stern’s estimate due to their using a higher field gradient. They had confirmed the Bohr-Sommerfeld theory! Gerlach sent a congratulatory postcard to Bohr!



Aftermath: It was generally acclaimed as a triumph of the “old” quantum theory (of course the “new” quantum theory or quantum mechanics or electron spin were yet to be discovered) and to confirm the reality of Bohr orbits, and converted the non-believers(including Stern). No one questioned why two instead of three lines…. nor raised any other questions!(not even Pauli, nor Heisenberg……. . etc). There was an interesting reaction from Einstein and Ehrenfest. In a paper written a few weeks after the publication of the S-G result, they raised a question: They did a semi-classical calculation of the time it would take the atom to change from one polarity to the other in the magnetic field by releasing the energy difference as radiation, and found a time of several hundred years. The puzzle was that the amount of time spent by the atoms in the magnet was much smaller, about 10 -4 s! They considered various possible explanations…. Although this was BEFORE Quantum Mechanics or the concept of a wave function; obviously it was an early example or premonition(? ) of wave function collapse!

Reactions to the Stern-Gerlach experiment and the result!: Sommerfeld: “Through their clever experimental arrangement Stern and Gerlach not only demostrated ad oculos the space quantization of atoms in a magnetic field but they also provided the quantum origin of electricity and its connection with atomic structure. ” Einstein: ”The most interesting acheivement at this point is the experiment of Stern and Gerlach. The alignment of the atoms without collisions via radiative excagnge is not comprehensible based on the current theoretical methods; it should take more than 100 years for the stoms to align. I have done a little calculation with Ehrenfest. Rubens considers the experimental result to be absolutely certain. ”

Franck: “More important is whether this proves the existence of space quantization. Please add a few words of explanation to your puzzle, such as what’s really going on. ” Bohr: ”I would be very grateful if you or Stern could let me know, in a few lines whether you interpret your experimental results in this way that the atoms are oriented parallel or opposed, but normal to the field, as one could provide theoretical reasons for the latter assertion. ” Pauli: ”This should convert even the nonbeliever Stern. ”

Rabi: ”As a beginning student back in 1923, I…. . hoped with ingenuity and innovativeness I could find ways to fit the atomic phenomena into some kind of mechanical system…. . My hope(to do that) died when I read about the Stern-Gerlach experiment…The results were astounding, although they were hinted at by quantum theory …This convinced me once and for all that an ingenious classical mechanism was out and we had to face the fact that quantum phenomena required a completely new orientation. ”

Incidentally, the fact of there being two, rather than three lines (expected in the Debye-Sommerfeld ) was not raised! The fact that the values for the magentic moment agreed with that expected for an orbit with l =1, and ml = +1 and -1 was accepted without question. The value for μ deduced from the observed splitting was exactly one Bohr magneton, namely eh/2 mc. As it turned out, the presence of two lines was due to two possibilities for the spin and the coincidence of the magnetic moment being the same was due to the factor of 2 cancelling out with the factor of ½! So although the Stern-Gerlach experiment was indeed the first observation of electron spin, it was credited as having confirmed space quantisation and Bohr-Sommerfeld model of the atom and NOT the electron spin! So NOT interpreted correctly!

B. Friedrich and D. Herschbach during a reenactment (2002) story of the bad cigar!

Plaque honoring Stern and Gerlach in

Invention of Electron Spin: By 1924 -5 the anomalous Zeeman effect, and generally the detailed spectra including the fine structure had become very confusing. There were proposals for ½ integral quantum numbers with empirical formulas such as the Lande g-factor, which was essentially data fitting. As early as 1921, Arthur Compton had raised the notion of a magnetic electron, which had spin. But the first serious proposal of electron spin was due to Ralph Kronig in 1925. He was just 21, and after finishing his Ph. D. at Columbia in NY, was joining Pauli as his assistant in Tubingen. When he arrived, he had the idea for electron spin with angular momentum of s=ħ/2 , which could take two values along any given direction, but with an associated magnetic moment μ = (ge ħ/2 mc)s, where g = 2, rather than 1 as it is in the case of orbital magnetic moment, to explain the anomalous Zeeman effect. Pauli was very negative and angry and rejected his proposal, and he got a similar reception from Heisenberg, Bohr etc. ; Kronig did not publish his idea and put his notes away. After the acceptance of Goudsmit-Uhlenbeck publication of the same idea, he published a summary of the objections! (μL = (e/2 mc)L, μS = g(e/2 mc)S)

Classical electron radius=r= e 2/mc 2 Compton radius of electron = r. C= ħ/mc= r /α where α= 1/137 is fine structure constant. To get the angular momentum =mvr to be h/2, the velocity at the periphery has to be = 1/2(c/α) = (137/2)c for r But only c/2 for r. C !

A few months later, Goudsmit and Uhlenbeck, students of Ehrenfest in Leiden had the same idea as Kronig! But in contrast to Pauli, Ehrenfest was very supportive, and told them to write it up and publish it! Both proposals of Kronig and of Goudsmit-Uhlenbeck suffered from a problem of “factor of two” as pointed out by Heisenberg. Namely, although the factor g had to be 2 to obtain the correct splitting in the anomaolous Zeeman effect (and in the Stern-Gerlach effect), g had to be 1 in the calculation of fine structure splitting to get the right value. When Ehrenfest took Goudsmit and Uhlenbeck to the Leiden train station to meet Einstein who was passing thru and told him about their work and the factor of two problem, Einstein said without a moment’ s thought that it must be a relativistic effect! As L. H. Thomas showed a few days later! One of Pauli’s objections was that a spinning sphere with the spin of (ħ/2) and a classical charge radius would have speeds larger than c at the periphery! But if the radius is Compton, it is not a problem….

n When did it become clear that the spin “invented” by Kronig, Goudsmit and Uhlenbeck in 1925 had already been observed in 1922 by Gerlach and Stern? : In 1927, two experiments were done by young graduate students in Urbana, Illinois and in Aberdeen, Scotland! R. G. J. Fraser measured the shape of hydrogen atom by scattering and found it to be spherically symmetric; T. E. Phipps and J. B. Taylor did a Stern-Gerlach experiment with hydrogen atoms and confirmed that they behave just like Silver atoms and split into two beams. They all concluded that the atoms were in the l=0, ground state of the Schrodinger wave function Ψ(1, 0, 0) and hence the Stern-Gerlach effect was entirely due to the spin of the single electron and had nothing to do with the socalled “space-quantization” or electron orbits in the atom. By then it was clear that silver and many other atoms with a closed shell and one electron in the outer shell had zero orbital angular momentum! This was the first clear statement that Stern. Gerlach had indeed observed the electron spin!…

By mid ‘ 30’s most textbooks (e. g. Slater, 1935, etc. ) explained the initial confusion and the final clarification giving full credit to Stern-Gerlach. But eventually there ceased to be any discussion of this detailed history in most textbooks except by philosophers and historians of science who continue to argue to this day……. about ontology and epistemology….

. So a discovery(Stern-Gerlach, 1922) became an invention(Goudsmit-Uhlenbeck, 1925) This is opposite of the usual/normal order: Invention followed by discovery e. g. Quarks invented in 1963(Gell-Mann-Zweig), discovered in 1968 -9(SLAC-MIT) Also “charm” invented in 1964(Bjorken-Glashow, Maki, Hara), discovered in 1976(SLAC-LBL) Hence the confusion……

The question , whether it is possible to do an inverse Stern-Gerlach experiment was raised by Wigner, and earlier by Bohm… n Actually what is meant is the following: suppose one does a S-G experiment and gets two beams one spin up along z and one spin down along z. Now take the spin up along z and do a second S-G experiment for spine along x. Then can one take the spin up along x and spin down along x and reconstruct the spin up along z beam?

One issue raised by Bohm in his 1952 book and later by Wigner(1963) was whether one could take the two split beams and do an “inverse” S-G experiment to restore the original beam? This was answered by Schwinger, Scully and Englert in a series of papers in 1988 -9. (answer: ”not easy, but not impossible!”). “Is Spin Coherence like Humpty-Dumpty? ” The answer is yes and no! Humpty-Dumpty sat on a wall, Humpty-Dumpty had a great fall; All the king’s horses and all the king’s men couldn’t put Humpty-Dumpty together again. The riddle has a solution : egg! In the case of spin, the egg can be put together but the egg-shell has some cracks! They found that in order to restore the original spin state to 1 part in 100(i. e. to 1 %) one needed accuracy of 1 part in 105!

Stern-Gerlach for free electrons? In the Solvay Conference in 1927, Bohr and Pauli argued that for free electrons, a S-G experiment to measure the magnetic moment was impossible, and furthermore, for free electrons the concept was meaningless! The argument was published by Mott in 1928. This was reproduced in textbooks such as Mott, Mott and Massey, and modern ones like Baym, Gottfried etc… and is generally accepted as dogma…. .


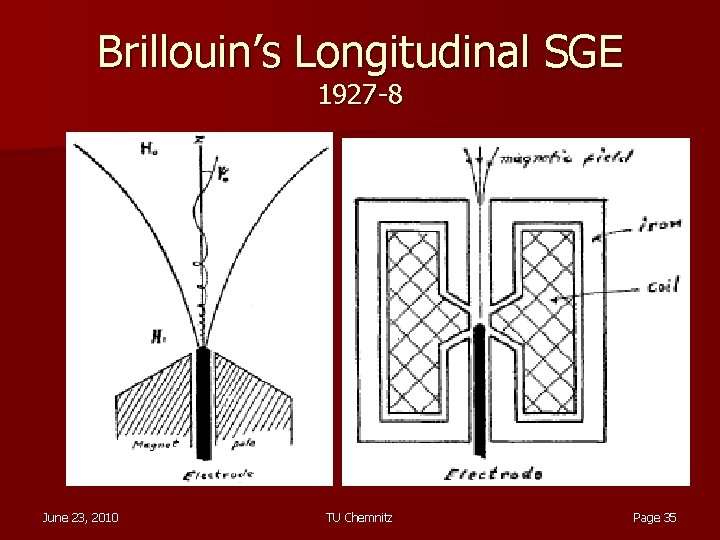
Brillouin’s Longitudinal SGE 1927 -8 June 23, 2010 TU Chemnitz Page 35

Brillouin’s Longitudinal SGE (2) Velocity along z changes as δB/δz changes until eventually coming to rest (α = insertion angle) very difficult, has never been tried!

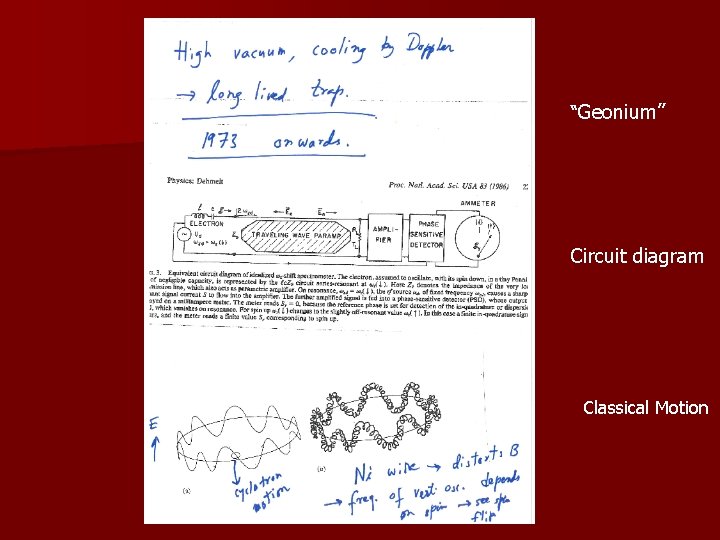
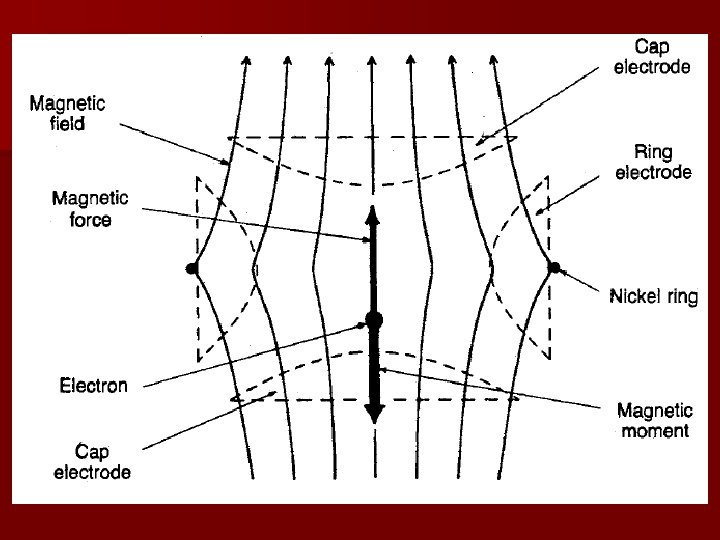
Enter Dehmelt! In the 1970’s, culminating in 1984, Dehmelt and his team in Seattle, started experiments with a Penning trap which seemed to do the impossible as defined by Bohr and Pauli! Penning trap is a device which traps charged particles using a combination of inhomogeneous electric quadrupole field an axial magnetic dipole fields. Dehmelt calls his way of measuring magnetic moment CSGE for Continuous Stern-Gerlach Experiment as opposed to the original one which is dubbed TSGE for Transient Stern-Gerlach Experiment!

“Geonium” Circuit diagram Classical Motion


CSGE – Schematic

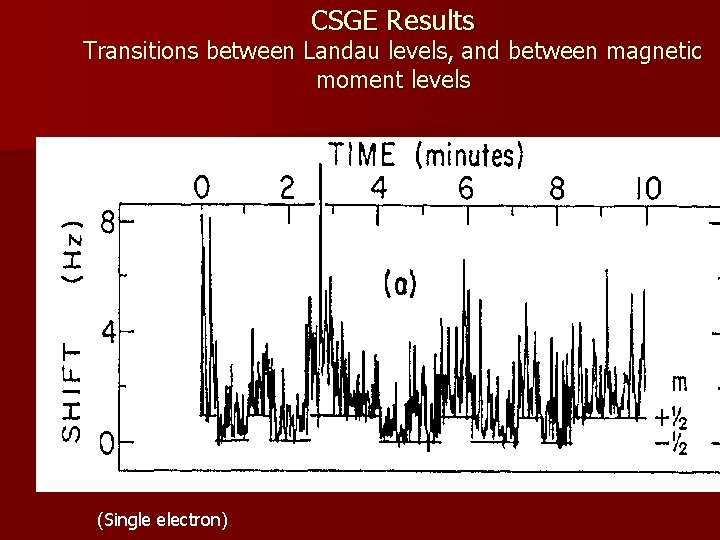
CSGE Results Transitions between Landau levels, and between magnetic moment levels (Single electron)

Dehmelt and co. measured the electron magnetic moment with fantastic accuracy: g/2= 1. 001159652188(4) To be compared to the QED prediction(e. g. Kinoshita) of: g/2= 1. 0011159652133(29) Leading to a Nobel Prize for Dehmelt (1989) and to admission by old-timers like Peierls who were around in the ‘ 20’s that “the electron is free in the sense intended by Bohr. This was one of the cases where Bohr was wrong. ” Although some philosophers of science may still not be convinced!

CSGE n Longitudinal, like Brillouin proposal n New detection scheme – frequency instead of observing changes in classical particle trajectories n Greatly increased detection sensitivity n Essentially free individual electron whose spin relaxation time is practically infinite

There is still some interest in trying to construct an experiment more like original SGE for free electrons or a la Brillouin proposal and attempts persist! Some recent attempts : Batelaan et al. , Phys. Rev. Lett. 79, 45(1997). G. H. Rutherford and R. Grobe, J. Phys. A 31, 9331(1998). B. M. Garraway and S. Stenholm, Phys. Rev. A 60, 63(1999) Turning back to Stern and his career…….

In 1932 -3, Stern decided to measure the magnetic moment of the proton, known also to have spin ½. He had to use clever tricks, as proton is also charged and would have Lorentz force. He chose neutral H 2 molecules which have two protons each. (In hydrogen atom, the 2000 times larger electron moment overwhelms that of the proton!) The trick was to choose parahydrogen, in which the proton spins are aligned so their moments add up, the electrons in ground state have total spin zero and orbital angular momentum also zero, The small correction due to non zero temperature and rotational motion is inferred from the study of ortho-hydrogen. So one measures twice the magnetic moment of the proton.

When he had announced his intention to do this measurement, he was berated and discouraged by theorists(including Pauli) for wasting his time since one “knew” the answer as it was given by Dirac equation, namely one nuclear magneton= eh/2 Mc As it turned out, he was vindicated when he found a value almost 3 times bigger than the expectation! Later on in the mid-fifties this large anomalous value led to some question about the existence of anti-proton since proton apparently did not obey Dirac equation!

Otto Stern(1888 -1969) – Note: Stern received the Nobel Prize in 1943 "for his contribution to the development of the molecular ray method and his discovery of the magnetic moment of the proton”(!). He also did following: – 1. Tested for the first time the Maxwell-Boltzmann distribution(speed as a function of the temperature) (1920) – 2. “discovered” electron spin albeit unknowingly!(1922) – 3. experimentally tested using diffraction the de Broglie relationship for many atoms and molecules for the first time(1926) – 4. measured the magnetic moment of the proton (1933)

Students, Assistants and “post-docs” of Stern who went on to win Nobel Prizes: Isidore Rabi, 1944, Felix Bloch, 1952, Polykarp Kusch, 1955, Emilio Segre, 1959, Norman Ramsey, 1989, The Numberf of nominations for Stern to receive the Nobel Prize: 82 He has been justly called: “The Founding Father of experimental atomic physics”

It can be claimed that descendents of the Stern. Gerlach experiment are legion…. . including nuclear magnetic resonance, optical pumping, atomic clocks, anomalous moments, and many other practical applications …… Subsequent time-line: In 1934, after the Nazi takeover, Stern moved to USA at the Carnegie Inst. Tech. after ensuring that all in his lab had found secure positions. At Carnegie with an ill-equipped lab he couldn’t perform any interesting expts. He retired to Berkeley in 1945, and stayed there until his death in 1969 As for Gerlach, he worked on radiometric pressure, and material science. In 1944, he became head of German Nuclear Research Program and was detained at the Farm Hall along with other German Physicists in 1945…

My takeaway from Stern’s career? It is OK to have theorists as friends, even be familiar with what they are talking about, but don’t take them too seriously, certainly don’t pay (too much) attention to their advice, and above all; any experiment that can be done is worth doing! (quote from Gerlach: ‘there is no such thing as an experiment that is too dumb!”) (Actually, he(Stern) was trained as a theorist, had many close theorist friends, including Pauli and would talk to him when he had problems!) Recently I discovered similar cautionary remarks by an ex DG (who shall remain unnamed) of CERN about the dangers of taking theorists too seriously …. .

References General reviews: 1. 2. 3. 4. 5. 6. B. Friedrich and D. Hercshbach, " How a Bad Cigar Helped Reorient Atomic Physics", Phys. Today, 56, 53(2003). J. P. Toennies et al. , ” Otto Stern (1888 -1969): The Founding Father of Experimental Atomic Physics”, ar. Xiv: 1109. 4864. The rest: N. Bohr, “On the Constitution of atoms and molecules”, I, II and III, Phil. Mag. 26, 1, 476, and 857(1913). A. Sommerfeld, Ann. der Physik, 51, 1(1916). A. Sommerfeld, Ann. der Physik, 491(1916). P. Debye, Physik. Zeits. 17, 507(1916). N. Bohr, "On the quantum theory of line spectra, Part II”, Mathematisk-Fyske Meddelsoer, Det Kgl. Danske Videnskabernes Selskab: Shrifter 8, 4. 1, 37(1918). A. Sommerfeld, “Atombau und Spektrallinien”, Braunschweig: Vieweg (1919).

7. 8. 9. 10. 11. 12. 13. 14. 15. 16. O. Stern, Zeit. fur Physik, 7, 249(1921). W. Gerlach and O. Stern, "Der Experimentalle Nachweis der Richtungsquantelung im Magnetfeld", Z. fur Physik. , 9, 349, 353(1922). A. Einstein and P. Ehrenfest, Zeit. fur Physik, 11 31(1922). A. Lande, Zeit. fur Physik, 5, 231, (1921), 7, 398(19210; 11, 353(1922). A. H. Compton, "the Magnetic Electron", J. Franklin Inst. , 192, 294(1921). S. Goudsmit and G. Uhlenbeck, Nature, 117, 264(1926). L. H. Thomas, Nature, 117, 5141926). R. L. Kronig, Nature, 117, 555(1926). T. E. Phipps and J. R. Taylor, Phys. Rev. 29, 309 (1927); R. G. J. Frazer, Proc. Roy. Soc. , A 114, 212(1927). M. O. Scully, J. Schwinger and B. G. Englert, "Is spin coherence like Humpty- Dumpty? " Phys. Rev. A 40,

17. N. Bohr, Collected Works, Vol. 6, J. Kalckar, ed. (N. Holland, Amsterdam, 1985), pp. 305 -350; W. Pauli, Proc. of the 6 th Solvay Conference, (Gauthier-Villars, Paris, 1932), p. 217, reprinted on p. 332 of above. 18. N. F. Mott, Proc. Roy. Soc. A 124, 425(1929). 19. N. F. Mott and H. S. W. Massey, “The Theory of Atomic Collisions”, Oxford, 1933 (1 st Ed. ). 20. K. Gottfried and T. M. Yan, “Quantum Mechanics”, Springer (2004), G. Baym, “Quantum Mechanics”, Benjamin (1969). 21. L. Brillouin, Proc. Acad. Nat. Sci. USA, 14, 155(1928). 22. H. Dehmelt, Proc. Natl. Acad. Sc. 83, 2291(1983). 23. O. Stern, Z. Physik. 2, 49(1920), 2, 417(1920). 24. I. Estermann, R. Frisch and O. Stern, Z. Phys. 73, 348(1931). 25. I. Estermann and O. Stern, 85, 17(1933).
 Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số.nguyên tố
Số.nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Kandinsky and klee experiment
Kandinsky and klee experiment Bromothymol blue lab
Bromothymol blue lab After the experiment scientists organize and the data
After the experiment scientists organize and the data Conrat and singer experiment
Conrat and singer experiment What is radial conduction
What is radial conduction Independent versus dependent variable
Independent versus dependent variable Thermic reaction
Thermic reaction Sheridan and king 1972 puppy experiment
Sheridan and king 1972 puppy experiment Temperature and solubility experiment
Temperature and solubility experiment Experiment control and variable
Experiment control and variable Hubel and wiesel experiment
Hubel and wiesel experiment Hubel and wiesel experiment
Hubel and wiesel experiment