The specific charge of the electron What is







- Slides: 12

The specific charge of the electron What is meant by specific charge? 2. Which equations are required to determine this? 3. Which experimental procedures can be used to determine the specific charge of an electron? 1.

1. 2. 3. 4. 5. 6. 7. Quizzle What is meant by the term specific charge (AKA charge to mass ratio)? Which particle has the largest specific charge? What will be deflected more in a magnetic field; a proton or an electron? What are the units of specific charge? What is the specific charge of a helium nucleus (alpha particle)? A sulphur atom has a nucleon number of 32. What is the specific charge of an S 2 - ion? How do you think this was determined by physicists in the good old days?

1. 2. 3. 4. 5. 6. 7. Answers e/m for an electron Electron, lowest charge Ckg-1 = 4. 85 × 107 C kg-1 = 6. 0 × 106 C kg-1 Fine beam tube type things

J. J. Thomson

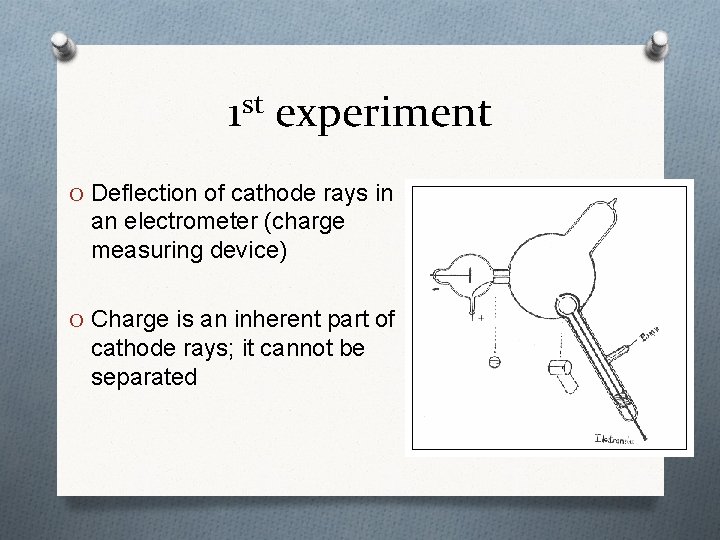
1 st experiment O Deflection of cathode rays in an electrometer (charge measuring device) O Charge is an inherent part of cathode rays; it cannot be separated

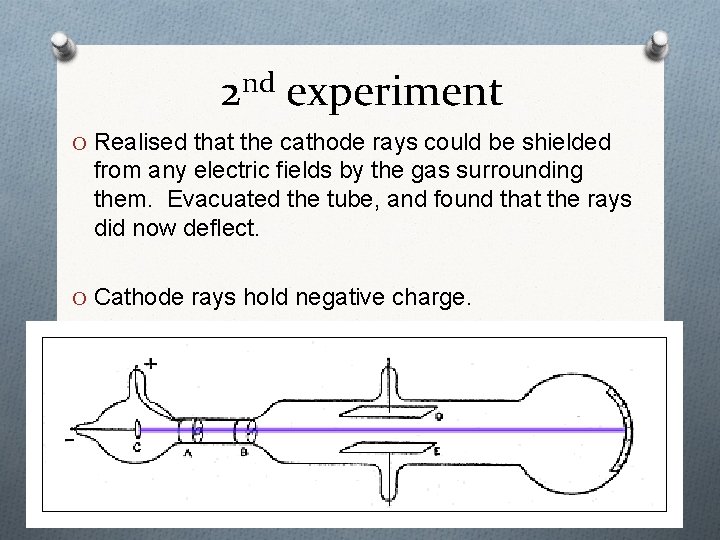
2 nd experiment O Realised that the cathode rays could be shielded from any electric fields by the gas surrounding them. Evacuated the tube, and found that the rays did now deflect. O Cathode rays hold negative charge.

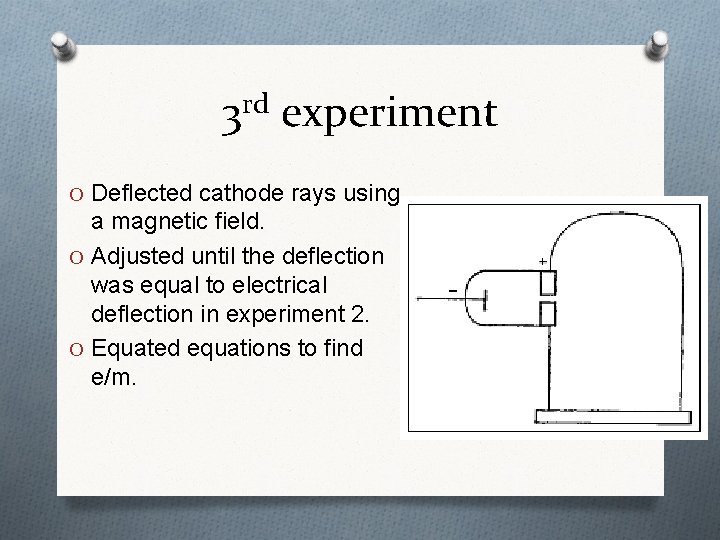
3 rd experiment O Deflected cathode rays using a magnetic field. O Adjusted until the deflection was equal to electrical deflection in experiment 2. O Equated equations to find e/m.

Significance O Thomson found the specific charge of e to be much greater than H+. O Thomson produced three hypotheses: 1. Cathode rays are charged particles (which he called "corpuscles"). 2. These corpuscles are constituents of the atom. 3. These corpuscles are the only constituents of the atom.

Fine beam tube

Measuring e/m with fine beam tube 1. Write an expression for the magnetic force on an electron and equate this to the centripetal force. 2. Write an expression equating the electron’s KE to the work done by the accelerating potential (VA is the anode p. d. ) 3. Eliminate the electron speed v from these equations to derive an expression for e/m in terms of VA, B (magnetic flux density), r (radius of curvature of beam)

Finding B

Question time 1. 2. 3. 4. 5. 6. How do the Helmholtz coils produce a circular trajectory for the electrons? Write an expression to show the magnetic force on an electron is equal to the centripetal force Rearrange this to give the specific charge What is the anode voltage of our fine beam tube? What is the specific charge on an electron? Using your answers to the previous questions, calculate the magnetic flux density produced by the Helmholtz coils E = F/Q, E = V/d, F = Bev, F = mv 2 /r, e. V = ½ mv 2
 Electron specific charge
Electron specific charge Electric charges of the same sign
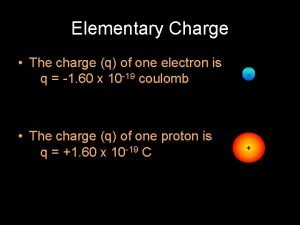
Electric charges of the same sign What is the charge of an electron
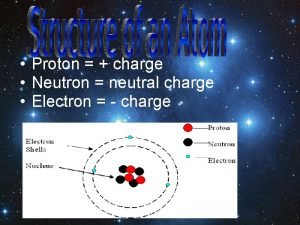
What is the charge of an electron Electron charge
Electron charge Electron volts to volts
Electron volts to volts Charge d'un électron
Charge d'un électron Thermionic emission experiment
Thermionic emission experiment Charge of electron
Charge of electron Isoelectronic species examples
Isoelectronic species examples Electron charge
Electron charge Electron configuration of na+1
Electron configuration of na+1 Difference between charge and electric charge
Difference between charge and electric charge Difference between charge and electric charge
Difference between charge and electric charge