The Solution Process What affects solubility Factors Affecting

The Solution Process What affects solubility?

Factors Affecting Solutions Surface area of solute Agitation Heat

Factors Affecting Solutions Solubility: maximum amount of solute that can be dissolved in a given quantity of solvent at a specific temperature Depends on: Type of solvent and solute (remember: like dissolves like!) Temperature Pressure (for gases)

Factors Affecting Solutions Solution equilibrium: eventually, equilibrium established between dissolution and crystallization Saturated: solution is at equilibrium Unsaturated: solution has less solute than equilibrium Supersaturated: special condition that is unstable

Factors Affecting Solutions Solubility: amount of substance that is required to form a saturated solution in a certain amount of solvent at a specific temperature Often given in tables or graphs See Table 13 -4 page 404
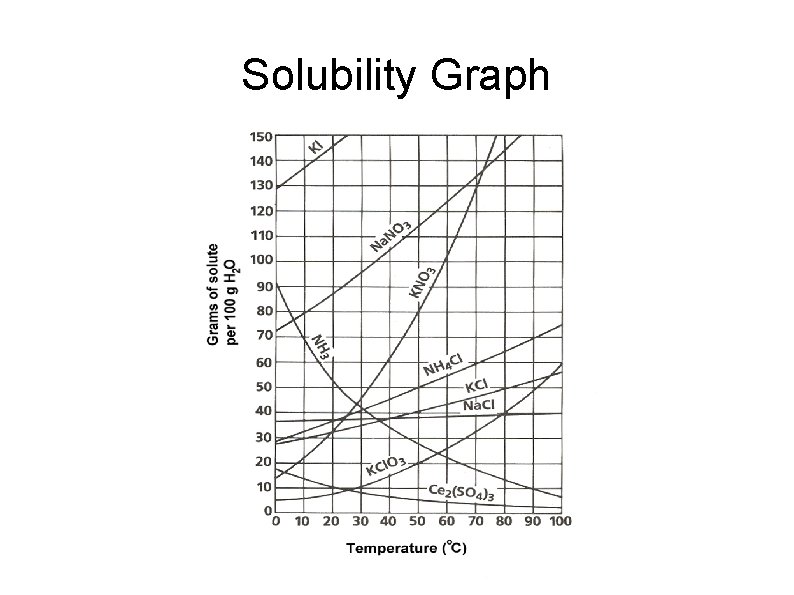
Solubility Graph

Factors Affecting Solutions Interactions between solute and solvent Strength of intermolecular forces Hydration: ionic solute is surrounded by water molecules Miscibility: liquid dissolves in another liquid Temperature: generally decreases solubility of gas, may increase solubility of a solid in liquid

Factors Affecting Gas Solutions Pressure: increase in pressure increases solubility of gas Henry's Law: Solubility of a gas in a liquid is directly proportional to the partial pressure of that gas on the surface of the liquid Effervescence: rapid escape of gas from a liquid in which it is dissolved
Heat of Solution Energy change during the process of solution The ΔHs is the amount of heat energy absorbed or released when a specific amount (mole) of solute dissolves in a solvent See Table 13 -5 p 410 Negative value means heat is released, positive means head is absorbed during solution

Remember! Making a solution involves three basic steps: Breaking the attraction between the solute particles (endothermic) Breaking the attraction between the solvent particles (endothermic) Forming attraction between solvent and solute (usually exothermic)
- Slides: 10