The Solid Phase l Inorganic components soil minerals











![Vermiculites & Micas 黑雲母(biotite) [(OH)4(Al 2 Si 6)Ⅳ(Mg. Fe)6ⅥO 20]2 -2 K+ Trioctahedral c-spacing Vermiculites & Micas 黑雲母(biotite) [(OH)4(Al 2 Si 6)Ⅳ(Mg. Fe)6ⅥO 20]2 -2 K+ Trioctahedral c-spacing](https://slidetodoc.com/presentation_image_h/b932cdc20b5f864428ecc969991a4d98/image-23.jpg)








- Slides: 40

The Solid Phase l. Inorganic components: soil minerals l. Organic components: soil organic matter l. Inorganic components: ØPrimary minerals: • The sand silt fractions consist largely of primary minerals. • Primary minerals are formed at elevated temperatures and inherited unchanged from igneous and metamorphic rocks, sometimes through a sedimentary cycle. • The most abundant primary minerals in soils: quartz(Si. O 2) and feldspars (MAl. Si 3 O 8) • Micas, pyroxenes, amphiboles, and olivine are in smaller quantities. 1

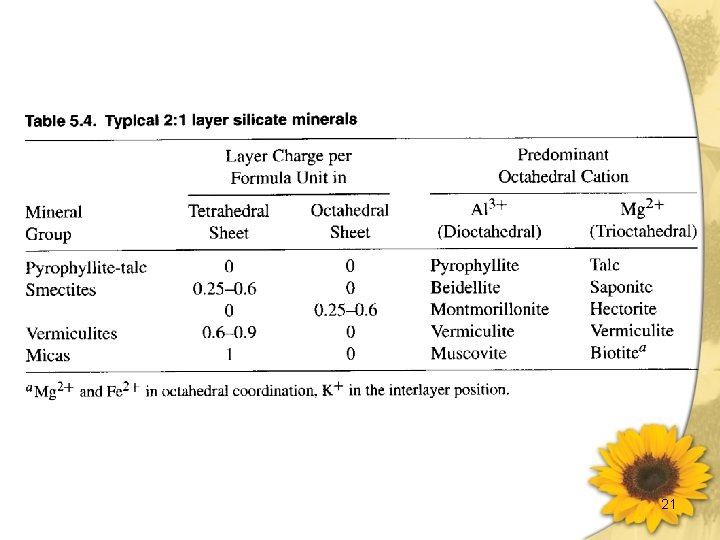
The Solid Phase(續) Ø Second minerals: • Minerals of the clay fraction of soils are largely secondary. • Secondary minerals are formed by low temperature reaction and either inherited from sedimentary rocks or formed directly by weathering. • Common secondary minerals in soils include the carbonate, the sulfur minerals, and the layer silicates, the various oxides. 2

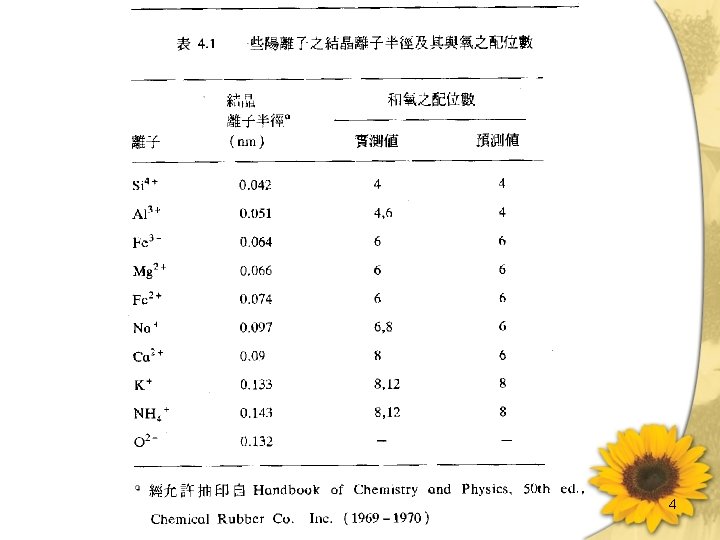
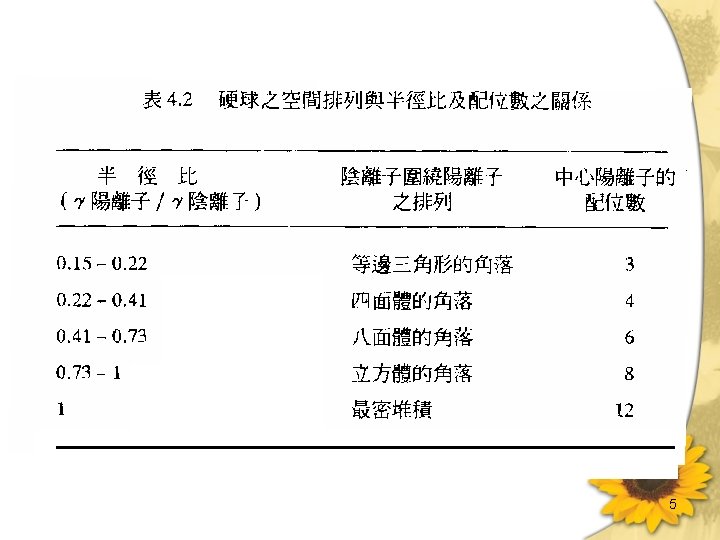
Crystal chemistry of silicates • Internal bonding in silicates is predominantly ionic. As a result, forces are undirected and ionic size plays an important part in determining crystal structure. 3

4

5

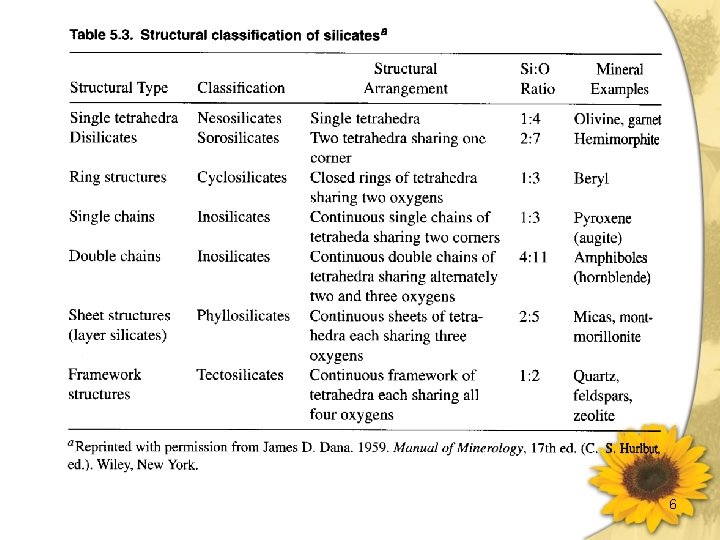
6

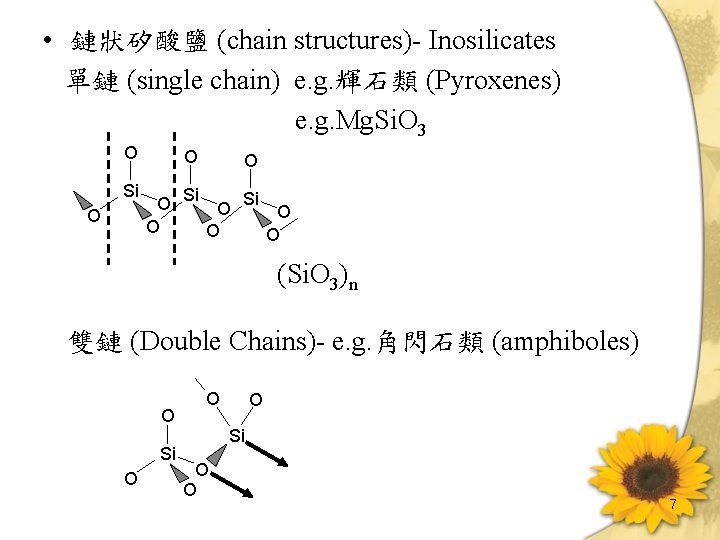
• 鏈狀矽酸鹽 (chain structures)- Inosilicates 單鏈 (single chain) e. g. 輝石類 (Pyroxenes) e. g. Mg. Si. O 3 O Si O O (Si. O 3)n 雙鏈 (Double Chains)- e. g. 角閃石類 (amphiboles) O Si O O 7

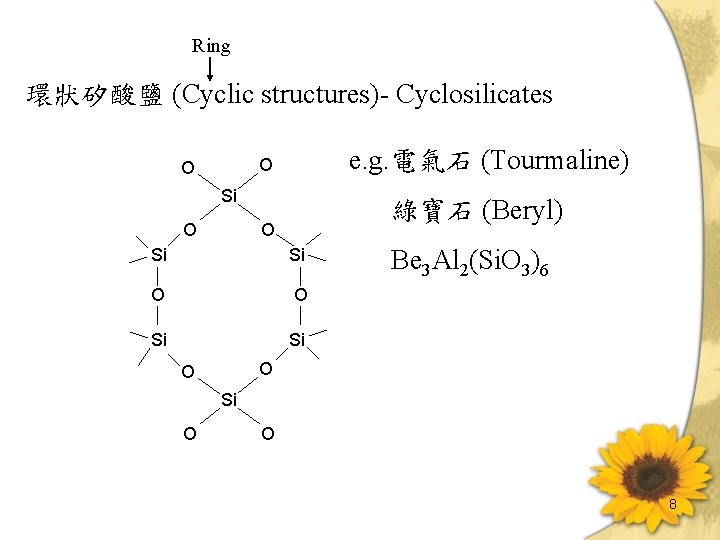
Ring 環狀矽酸鹽 (Cyclic structures)- Cyclosilicates e. g. 電氣石 (Tourmaline) O O Si O 綠寶石 (Beryl) O Si Si O O Si Si Be 3 Al 2(Si. O 3)6 O O Si O O 8

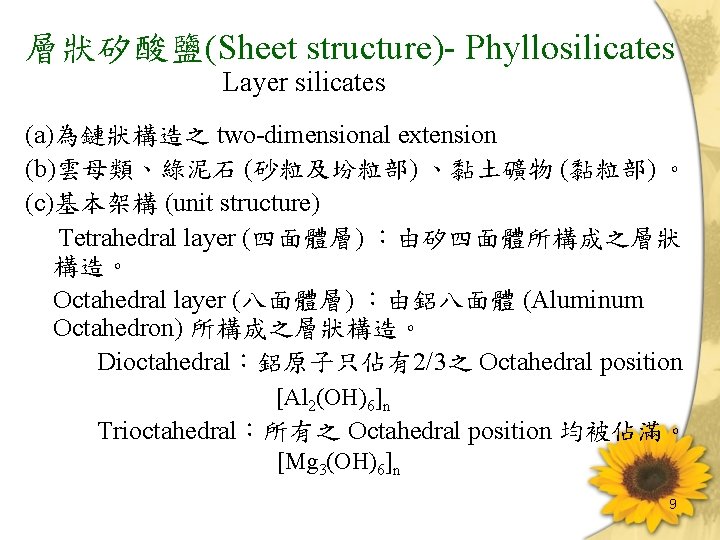
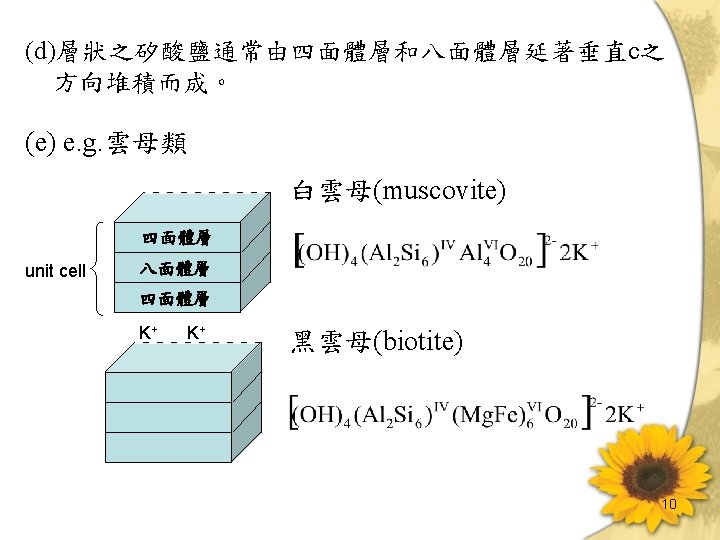
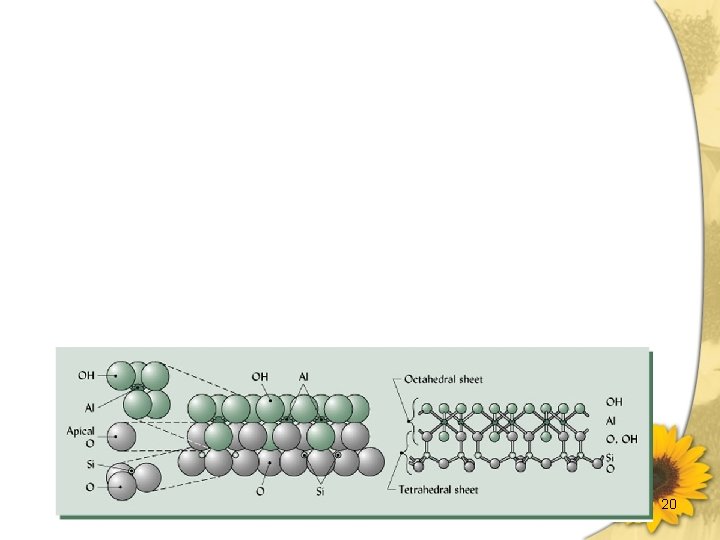
層狀矽酸鹽(Sheet structure)- Phyllosilicates Layer silicates (a)為鏈狀構造之 two-dimensional extension (b)雲母類、綠泥石 (砂粒及坋粒部) 、黏土礦物 (黏粒部) 。 (c)基本架構 (unit structure) Tetrahedral layer (四面體層) :由矽四面體所構成之層狀 構造。 Octahedral layer (八面體層) :由鋁八面體 (Aluminum Octahedron) 所構成之層狀構造。 Dioctahedral:鋁原子只佔有2/3之 Octahedral position [Al 2(OH)6]n Trioctahedral:所有之 Octahedral position 均被佔滿。 [Mg 3(OH)6]n 9


11


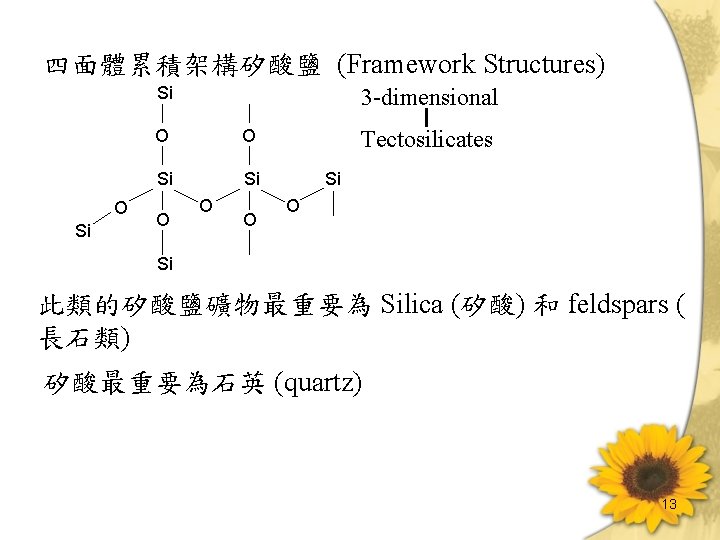
四面體累積架構矽酸鹽 (Framework Structures) Si O Si 3 -dimensional O O Si Si O O O Tectosilicates Si O Si 此類的矽酸鹽礦物最重要為 Silica (矽酸) 和 feldspars ( 長石類) 矽酸最重要為石英 (quartz) 13


• Crystal: an arrangement of ions or atoms that is repeated at regular intervals in three dimensions. • Unit cell: the smallest repeating three-dimensional array of a crystal. • Formula unit: The chemical composition of layer silicate minerals is normally expressed as one half of a unit cell in order to simplify the chemical formulas. Formula unit=1/2 unit cell 15

16

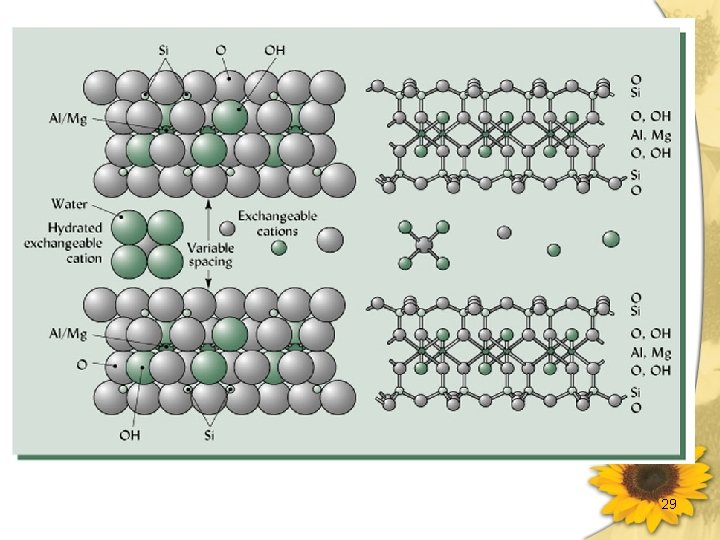
• Layer Charge The magnitude of charge per formula unit, when balanced by cations external to the unit layer. 17

高嶺石類 (kaolins) 1: 1 layer silicates 1. 基本構造 1: 1 層狀 (由一四面體層和八面體層共用 一些 O 原子而形成) 2. Unit Formula 組成 Si 2ⅣO 5 Al 2Ⅵ(OH)4 3. Lamella 間以 H-bonding 鍵結 c-spacing = 7. 2Å (0. 72 nm) 18


20

21

22
![Vermiculites Micas 黑雲母biotite OH4Al 2 Si 6ⅣMg Fe6ⅥO 202 2 K Trioctahedral cspacing Vermiculites & Micas 黑雲母(biotite) [(OH)4(Al 2 Si 6)Ⅳ(Mg. Fe)6ⅥO 20]2 -2 K+ Trioctahedral c-spacing](https://slidetodoc.com/presentation_image_h/b932cdc20b5f864428ecc969991a4d98/image-23.jpg)
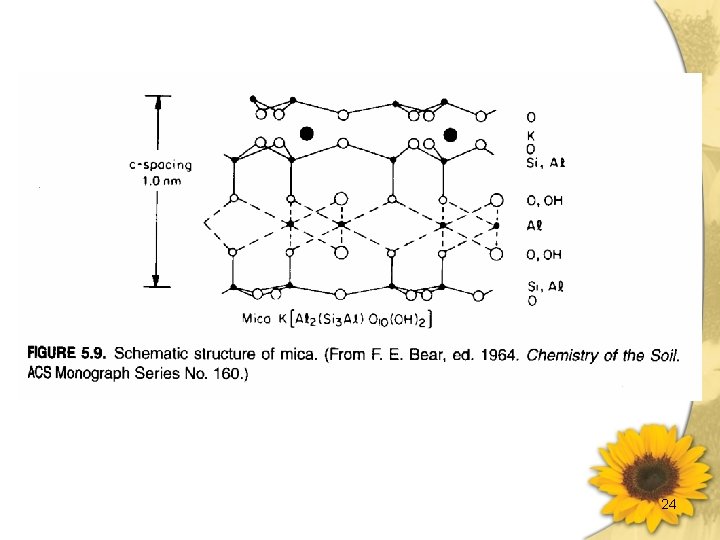
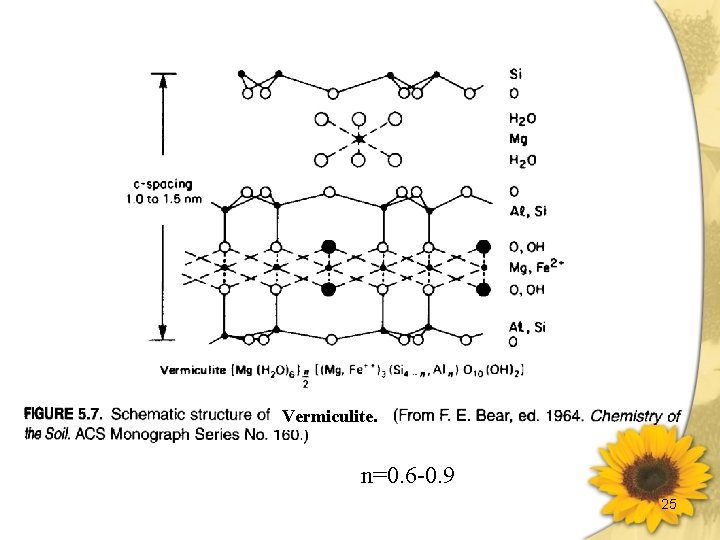
Vermiculites & Micas 黑雲母(biotite) [(OH)4(Al 2 Si 6)Ⅳ(Mg. Fe)6ⅥO 20]2 -2 K+ Trioctahedral c-spacing ~ 1. 0 nm CEC: 200 ~ 400 m moles (+) ·kg-1 Surface Area: 70 ~120× 103 m 3·kg-1 白雲母(muscovite) [(OH)4(Al 2 Si 6)ⅣAl 4ⅥO 20]2 -2 K+ Dioctahedral c-spacing 1. 0 nm CEC: 200 ~ 400 m moles (+) ·kg-1 Surface Area: 70 ~120× 103 m 3·kg-1 蛭石(vermiculite) [(OH)4(Alx. Si 8 -x)Ⅳ(Mg. Fe)6ⅥO 20]x-·Mg 2+ , X<2 CEC: 1200 ~ 1500 m moles (+) ·kg-1 c-spacing ~ 1. 4 -1. 5 nm 加熱脫水後 1. 0 nm 有限度膨脹之礦物 較雲母類有較少之負電荷,因同位置換少。 有較高含量之結晶水。 伊來石(illite) hydrous mica [(OH)4(Alx. Si 8 -x)ⅣAl 4ⅥO 20]x-K+ c-spacing 1. 0 nm H 3 O + 層間鍵結較白雲母弱,層之排列較不規則。 23

24

Vermiculite. n=0. 6 -0. 9 25


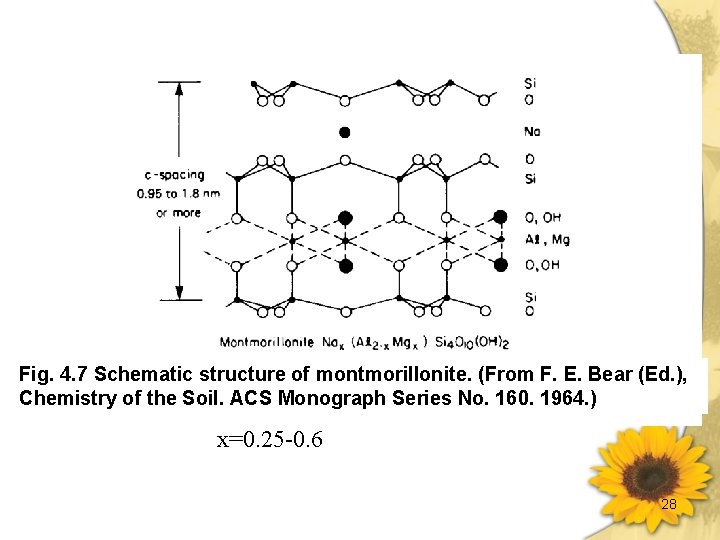
蒙特石類 (Smectites) (續) 5. 比表面積相當大(有相當大之內表面積)。 6. Layer charge 0. 25~0. 6 per formula unit. 7. 在土壤中常見有蒙特石(Montmorillonite、 Beidellite 和 Nontronite。 CEC: 800 ~ 1200 m moles (+)·kg -1 Surface Area: 600 - 800× 103 m 2·kg -1 27

Fig. 4. 7 Schematic structure of montmorillonite. (From F. E. Bear (Ed. ), Chemistry of the Soil. ACS Monograph Series No. 160. 1964. ) x=0. 25 -0. 6 28

29

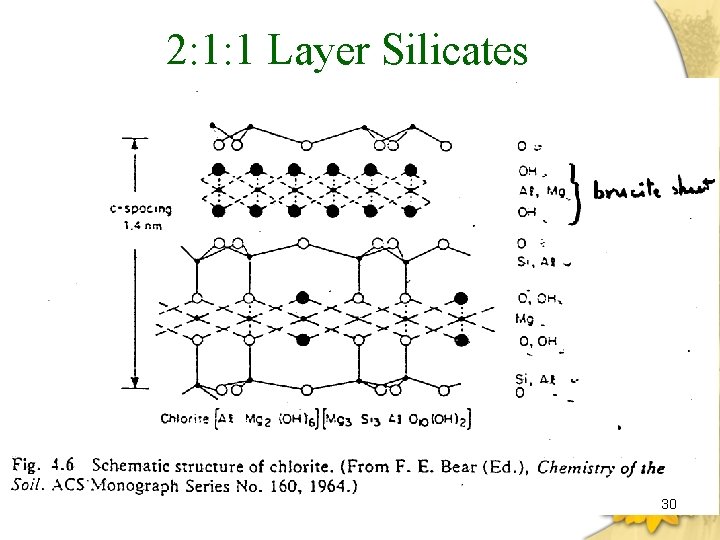
2: 1: 1 Layer Silicates 30




34


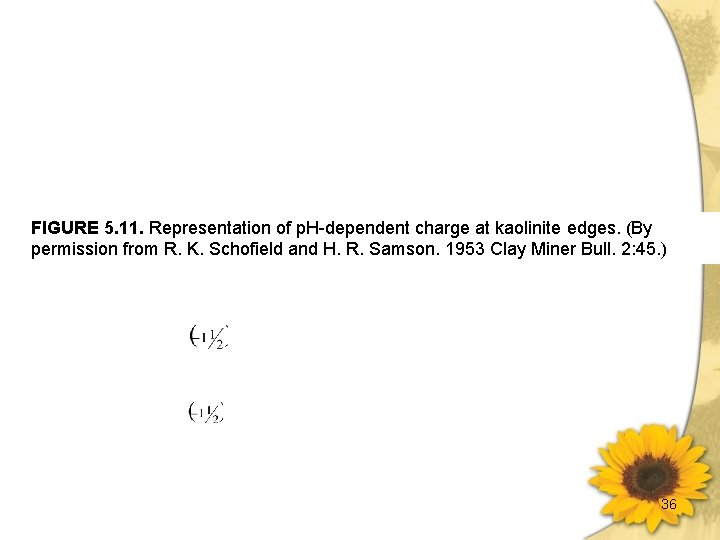
FIGURE 5. 11. Representation of p. H-dependent charge at kaolinite edges. (By permission from R. K. Schofield and H. R. Samson. 1953 Clay Miner Bull. 2: 45. ) 36

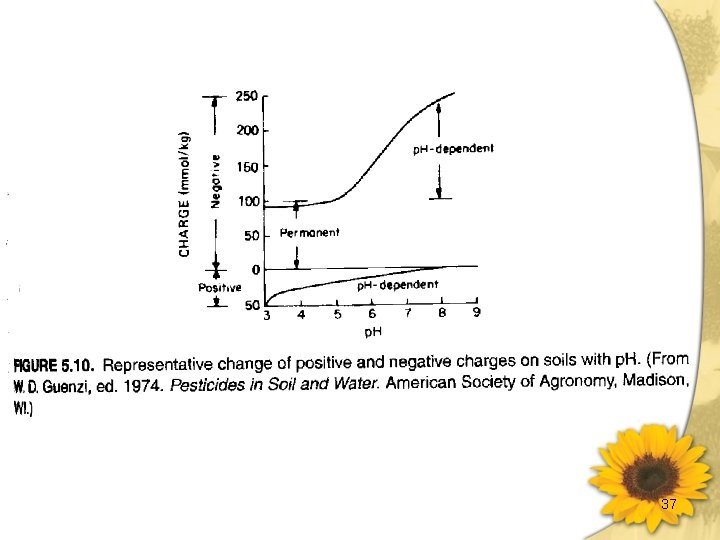
37

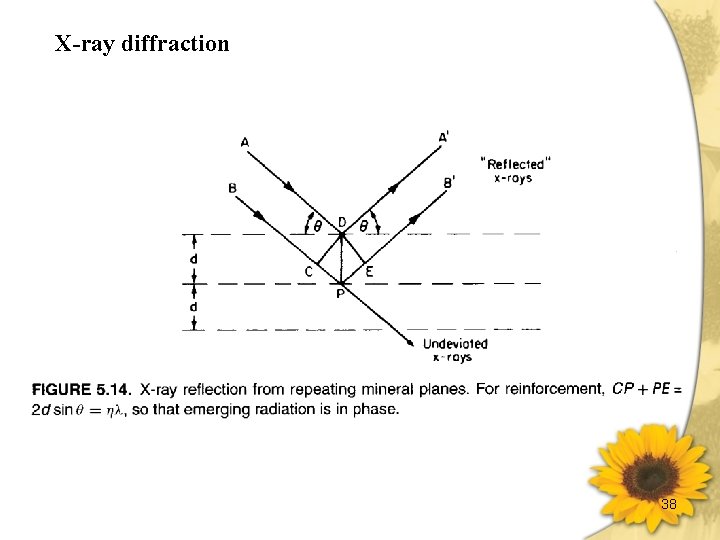
X-ray diffraction 38

Surface Area Measurements • Water vapor adsorption – internal & External • N 2 -gas adsorption (BET Eq. ) – External • Retention of polar molecules – internal & external Ø Ethylene glycol Ø glycerol Ø EGME (ethylene glycol monoethyl ether) 39

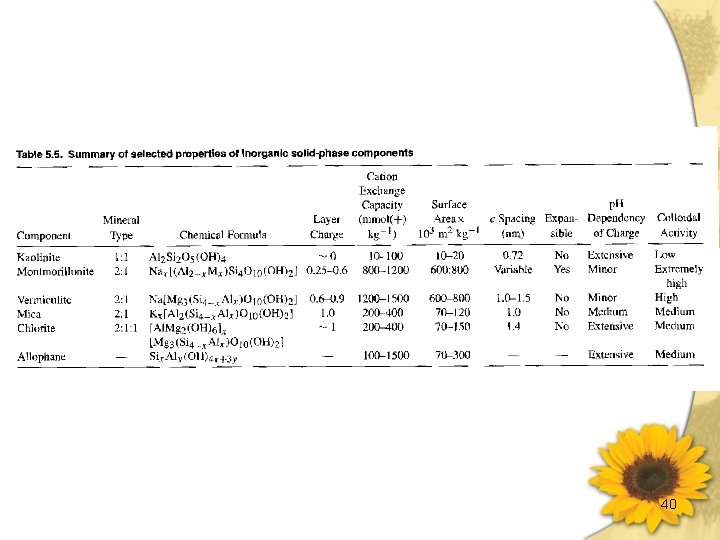
40