The Soil Reaction Soil Acidity and Alkalinity ILMU











- Slides: 43

The Soil Reaction Soil Acidity and Alkalinity ILMU TANAH 2016

Soil p. H * p. H - the negative log of the hydrogen ion(H+) concentration in the soil water solution. p. H = - log [ H+] * the p. H scale is how we measure acidity and alkalinity of solutions. at neutral (p. H =7) the number of H+ = OHRemember – at p. H of 6 there are 10 x more H+ ions than at a p. H 7 and there are 100 x more H+ ions between p. H 7 & 5

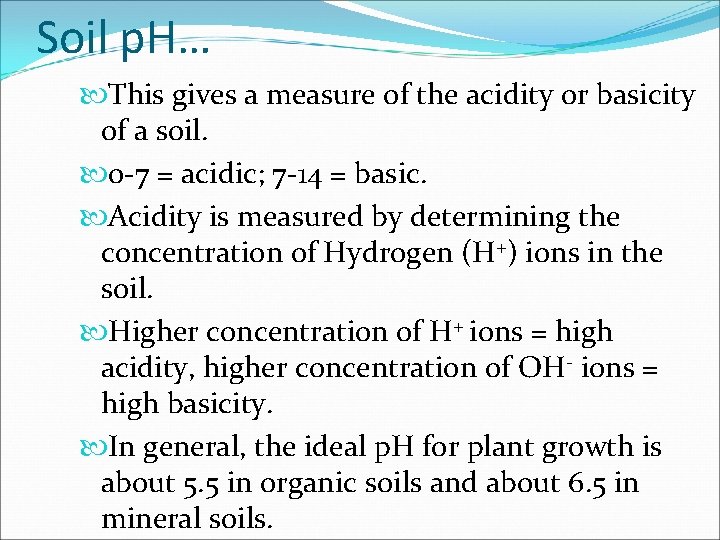
Soil p. H… This gives a measure of the acidity or basicity of a soil. 0 -7 = acidic; 7 -14 = basic. Acidity is measured by determining the concentration of Hydrogen (H+) ions in the soil. Higher concentration of H+ ions = high acidity, higher concentration of OH- ions = high basicity. In general, the ideal p. H for plant growth is about 5. 5 in organic soils and about 6. 5 in mineral soils.

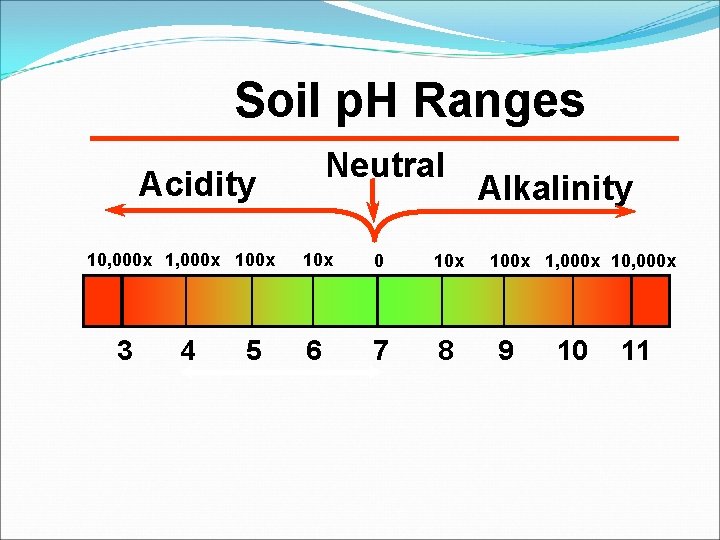
Soil p. H Ranges Neutral Acidity 10, 000 x 100 x 3 4 5 10 x 0 10 x 6 7 8 Alkalinity 100 x 1, 000 x 10, 000 x 9 10 11

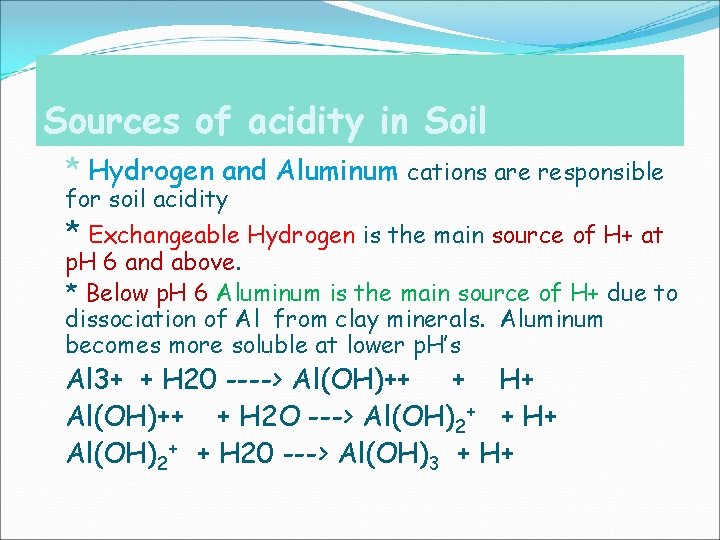
Sources of acidity in Soil * Hydrogen and Aluminum cations are responsible for soil acidity * Exchangeable Hydrogen is the main source of H+ at p. H 6 and above. * Below p. H 6 Aluminum is the main source of H+ due to dissociation of Al from clay minerals. Aluminum becomes more soluble at lower p. H’s Al 3+ + H 20 ----> Al(OH)++ + H+ Al(OH)++ + H 2 O ---> Al(OH)2+ + H+ Al(OH)2+ + H 20 ---> Al(OH)3 + H+

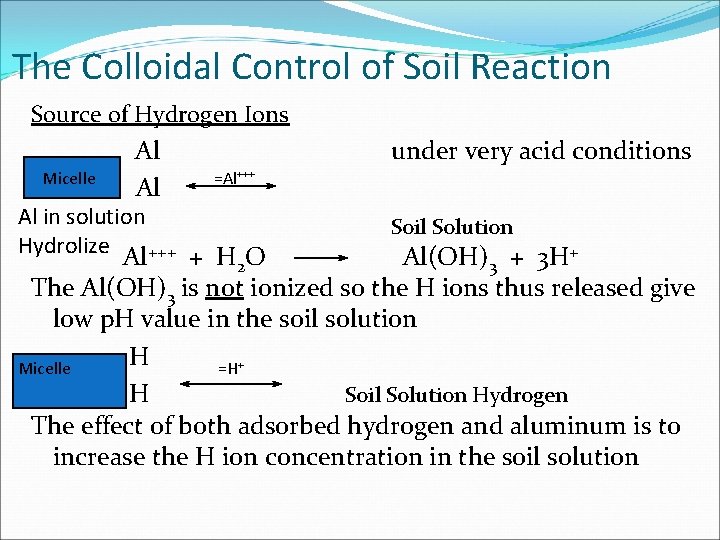
The Colloidal Control of Soil Reaction Source of Hydrogen Ions Micelle Al Al Al in solution Hydrolize +++ under very acid conditions =Al+++ Soil Solution Al + H 2 O Al(OH)3 + 3 H+ The Al(OH)3 is not ionized so the H ions thus released give low p. H value in the soil solution H Micelle =H+ H Soil Solution Hydrogen The effect of both adsorbed hydrogen and aluminum is to increase the H ion concentration in the soil solution

The Colloidal Control of Soil Reaction Source of OH ions Ca H Micelle + H 2 O Micelle + Ca++ + 2 OH Ca H Under natural conditions the reaction to furnish H and OH ions to the soil solution occur simultaneously

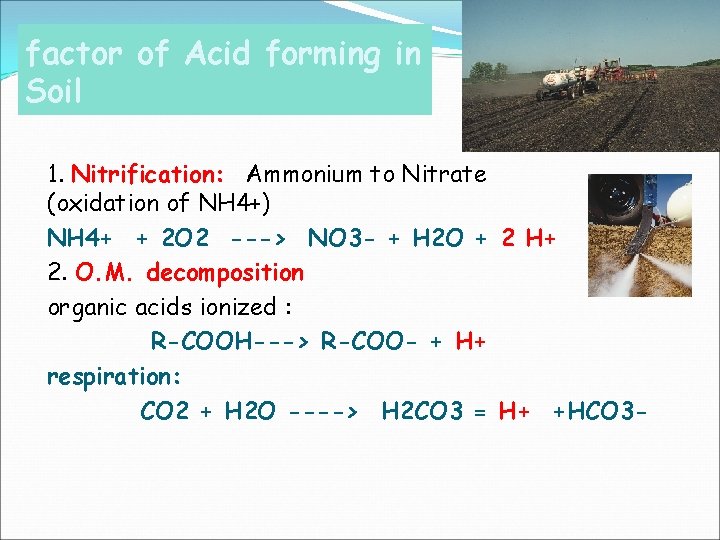
factor of Acid forming in Soil 1. Nitrification: Ammonium to Nitrate (oxidation of NH 4+) NH 4+ + 2 O 2 ---> NO 3 - + H 2 O + 2 H+ 2. O. M. decomposition organic acids ionized : R-COOH---> R-COO- + H+ respiration: CO 2 + H 2 O ----> H 2 CO 3 = H+ +HCO 3 -

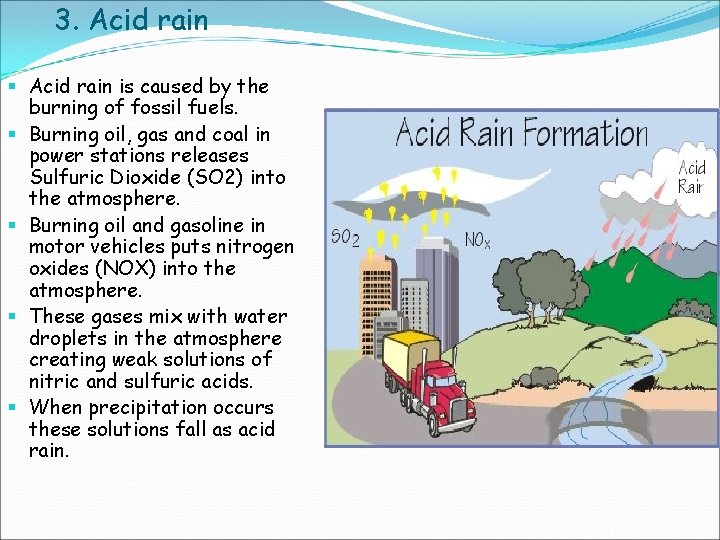
3. Acid rain § Acid rain is caused by the burning of fossil fuels. § Burning oil, gas and coal in power stations releases Sulfuric Dioxide (SO 2) into the atmosphere. § Burning oil and gasoline in motor vehicles puts nitrogen oxides (NOX) into the atmosphere. § These gases mix with water droplets in the atmosphere creating weak solutions of nitric and sulfuric acids. § When precipitation occurs these solutions fall as acid rain.

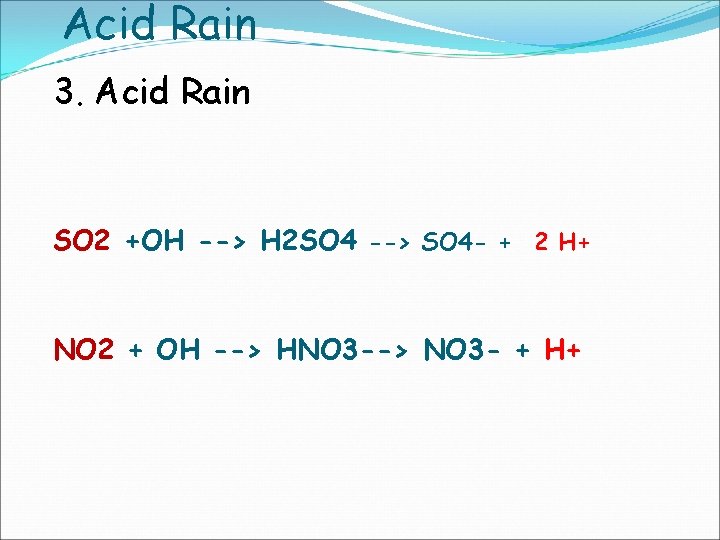
Acid Rain 3. Acid Rain SO 2 +OH --> H 2 SO 4 --> SO 4 - + 2 H+ NO 2 + OH --> HNO 3 --> NO 3 - + H+

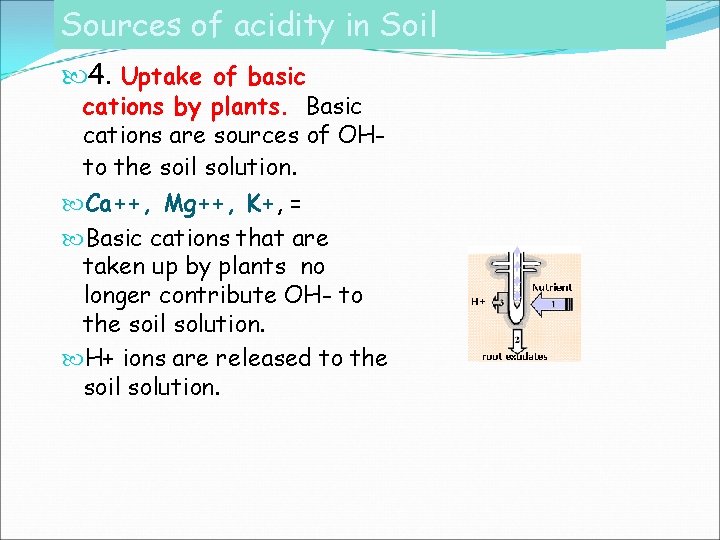
Sources of acidity in Soil 4. Uptake of basic cations by plants. Basic cations are sources of OHto the soil solution. Ca++, Mg++, K+, = Basic cations that are taken up by plants no longer contribute OH- to the soil solution. H+ ions are released to the soil solution.

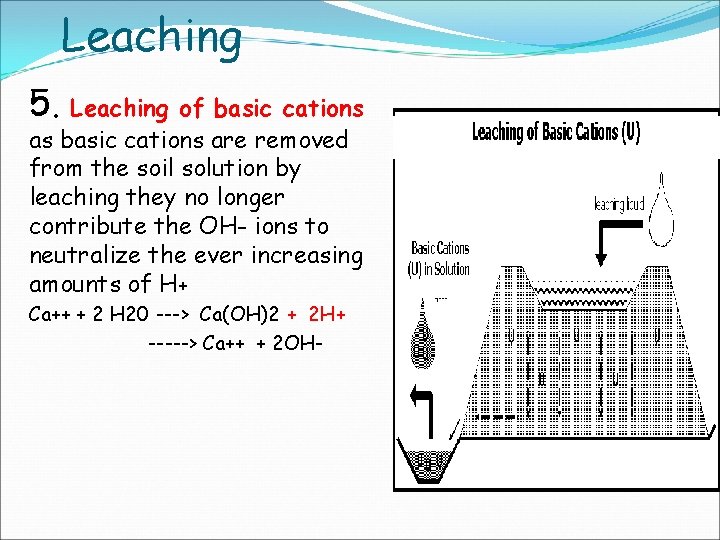
Leaching 5. Leaching of basic cations as basic cations are removed from the soil solution by leaching they no longer contribute the OH- ions to neutralize the ever increasing amounts of H+ Ca++ + 2 H 20 ---> Ca(OH)2 + 2 H+ -----> Ca++ + 2 OH-

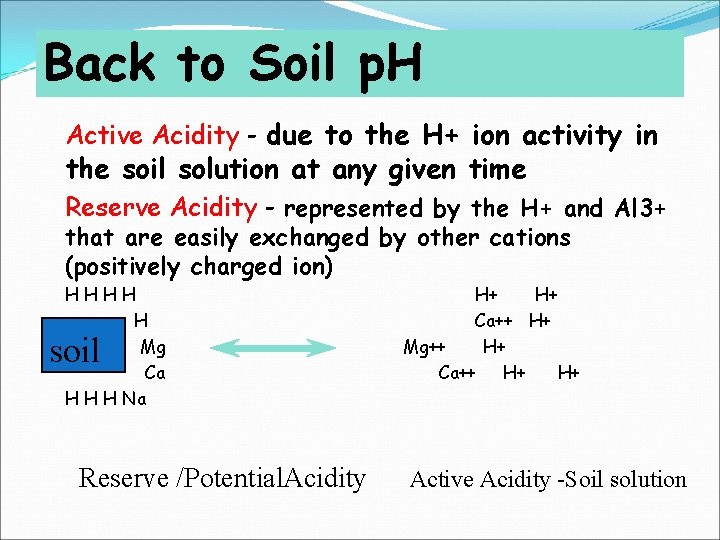
Back to Soil p. H Active Acidity - due to the H+ ion activity in the soil solution at any given time Reserve Acidity - represented by the H+ and Al 3+ that are easily exchanged by other cations (positively charged ion) HHHH H Mg Ca H H H Na soil Reserve /Potential. Acidity H+ H+ Ca++ H+ Mg++ H+ Ca++ H+ H+ Active Acidity -Soil solution

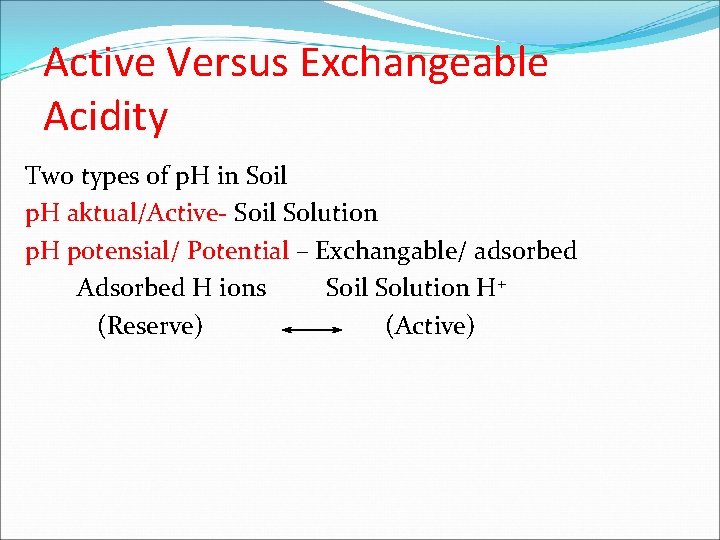
Active Versus Exchangeable Acidity Two types of p. H in Soil p. H aktual/Active- Soil Solution p. H potensial/ Potential – Exchangable/ adsorbed Adsorbed H ions Soil Solution H+ (Reserve) (Active)

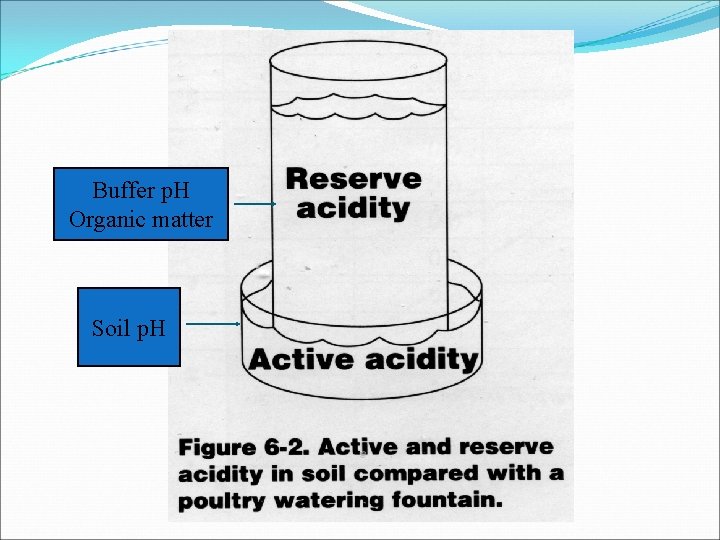
Buffer p. H Organic matter Soil p. H

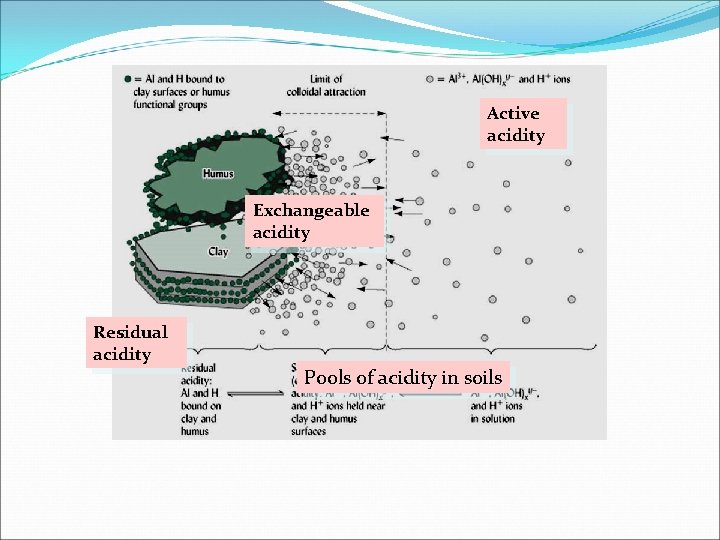
Active acidity Exchangeable acidity Residual acidity Pools of acidity in soils


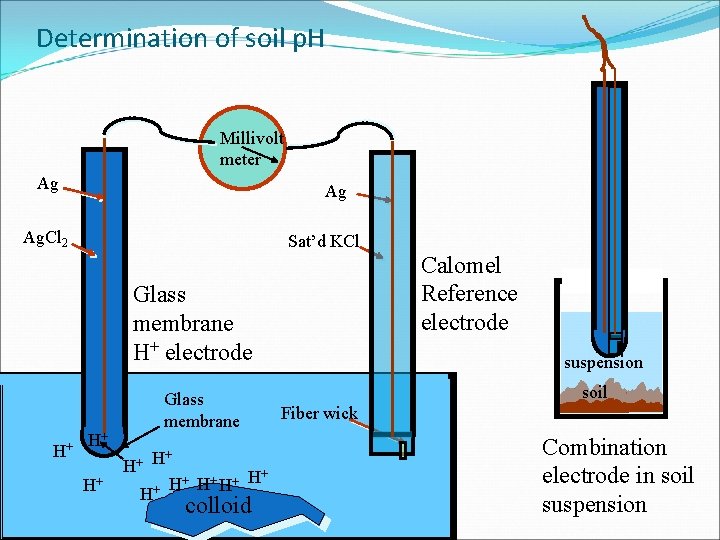
Determination of soil p. H Millivolt meter Ag Ag Ag. Cl 2 Sat’d KCl Calomel Reference electrode Glass membrane H+ electrode H+ H+ H+ Glass membrane H+ + + H H H H+ H+ colloid suspension soil Fiber wick Combination electrode in soil suspension

Combination p. H electrode

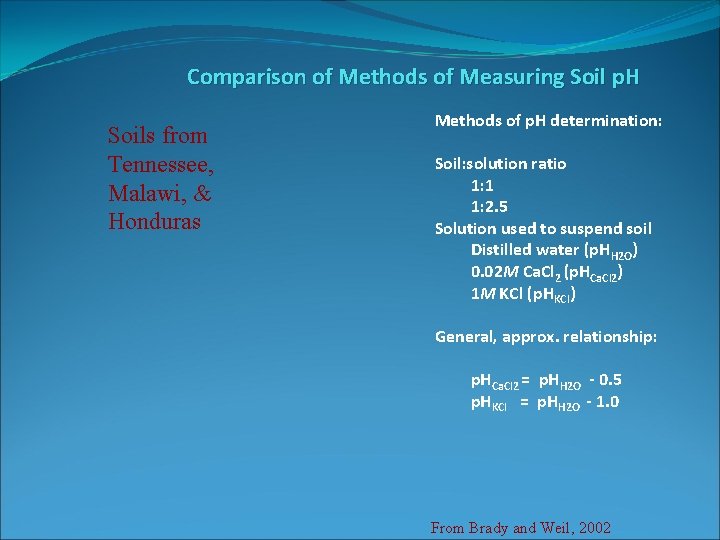
Comparison of Methods of Measuring Soil p. H Soils from Tennessee, Malawi, & Honduras Methods of p. H determination: Soil: solution ratio 1: 1 1: 2. 5 Solution used to suspend soil Distilled water (p. HH 2 O) 0. 02 M Ca. Cl 2 (p. HCa. Cl 2) 1 M KCl (p. HKCl) General, approx. relationship: p. HCa. Cl 2 = p. HH 2 O - 0. 5 p. HKCl = p. HH 2 O - 1. 0 From Brady and Weil, 2002

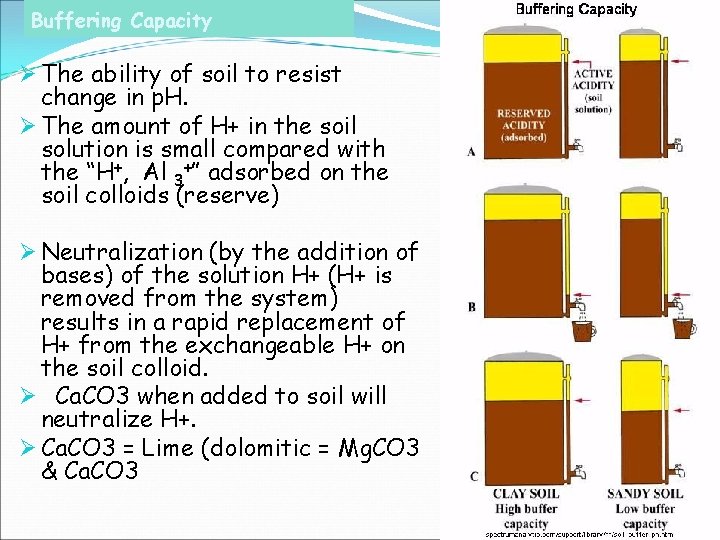
Soil Buffering Capacity… The tendency of soils is to resist changes of the p. H of the soil solution. This resistance is termed “buffering”. Soils have different buffering capacities. Generally, higher CEC = greater buffering capacity. Buffering capacity indicates dynamic equilibrium of soil solution. Changes of all types tend to be resisted by the system.

Buffering Capacity Ø The ability of soil to resist change in p. H. Ø The amount of H+ in the soil solution is small compared with the “H+, Al 3+” adsorbed on the soil colloids (reserve) Ø Neutralization (by the addition of bases) of the solution H+ (H+ is removed from the system) results in a rapid replacement of H+ from the exchangeable H+ on the soil colloid. Ø Ca. CO 3 when added to soil will neutralize H+. Ø Ca. CO 3 = Lime (dolomitic = Mg. CO 3 & Ca. CO 3

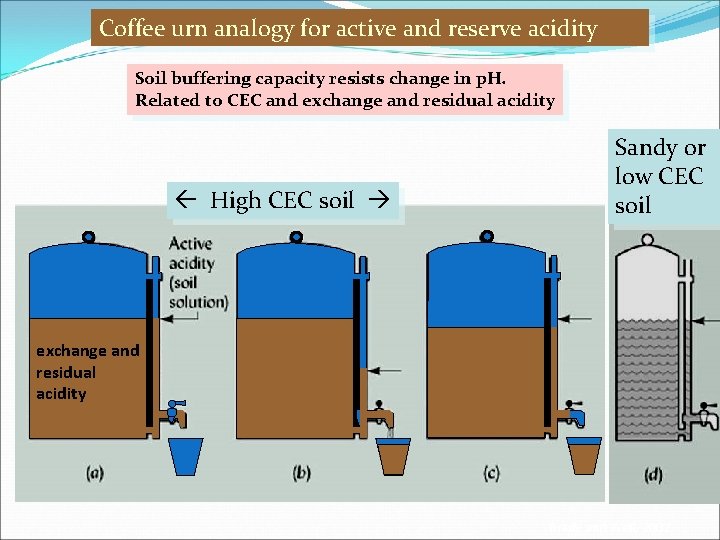
Coffee urn analogy for active and reserve acidity Soil buffering capacity resists change in p. H. Related to CEC and exchange and residual acidity High CEC soil Sandy or low CEC soil exchange and residual acidity Brady and Weil, 2002

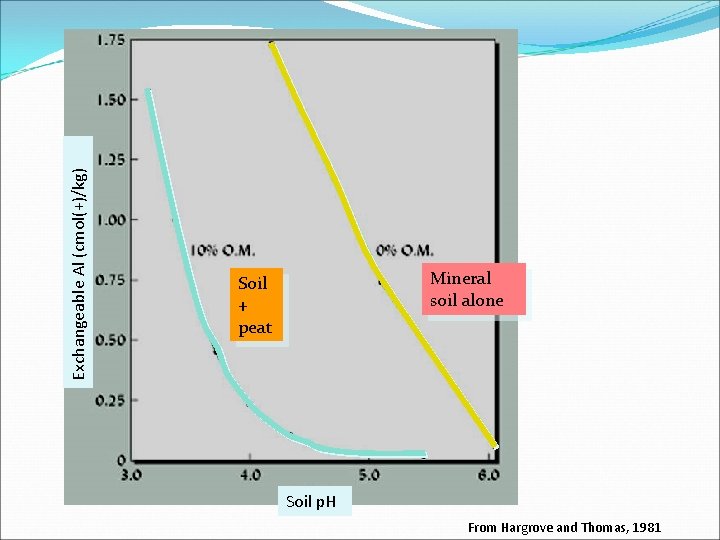
Exchangeable Al (cmol(+)/kg) Mineral soil alone Soil + peat Soil p. H From Hargrove and Thomas, 1981

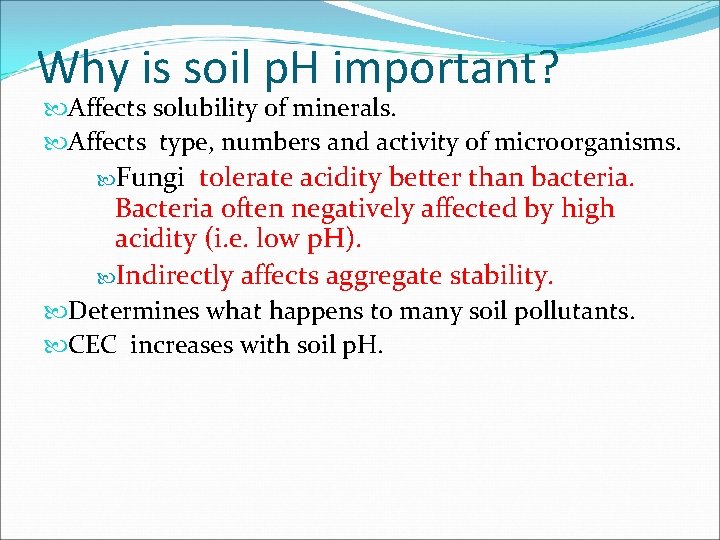
Why is soil p. H important? Affects solubility of minerals. Affects type, numbers and activity of microorganisms. Fungi tolerate acidity better than bacteria. Bacteria often negatively affected by high acidity (i. e. low p. H). Indirectly affects aggregate stability. Determines what happens to many soil pollutants. CEC increases with soil p. H.

Acid Soil “Headache” Aluminum toxicity Manganese toxicity Calcium deficiency Fe deficiency induced by Al Molybdenum deficiency Magnesium deficiency

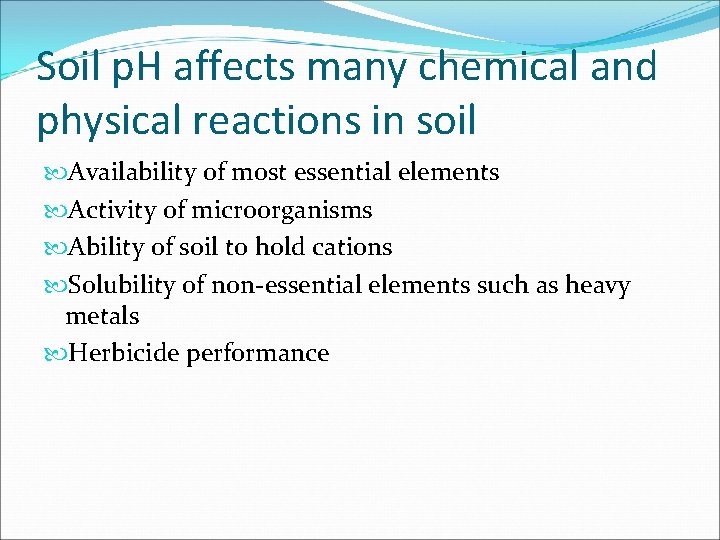
Soil p. H affects many chemical and physical reactions in soil Availability of most essential elements Activity of microorganisms Ability of soil to hold cations Solubility of non-essential elements such as heavy metals Herbicide performance

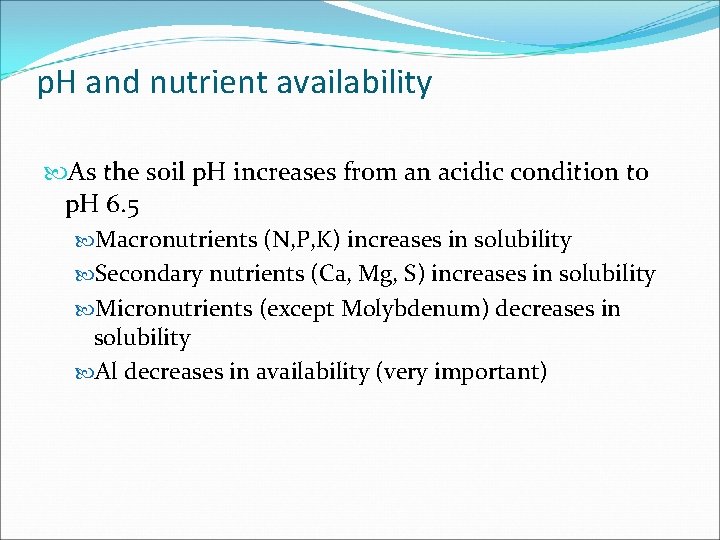
p. H and nutrient availability As the soil p. H increases from an acidic condition to p. H 6. 5 Macronutrients (N, P, K) increases in solubility Secondary nutrients (Ca, Mg, S) increases in solubility Micronutrients (except Molybdenum) decreases in solubility Al decreases in availability (very important)

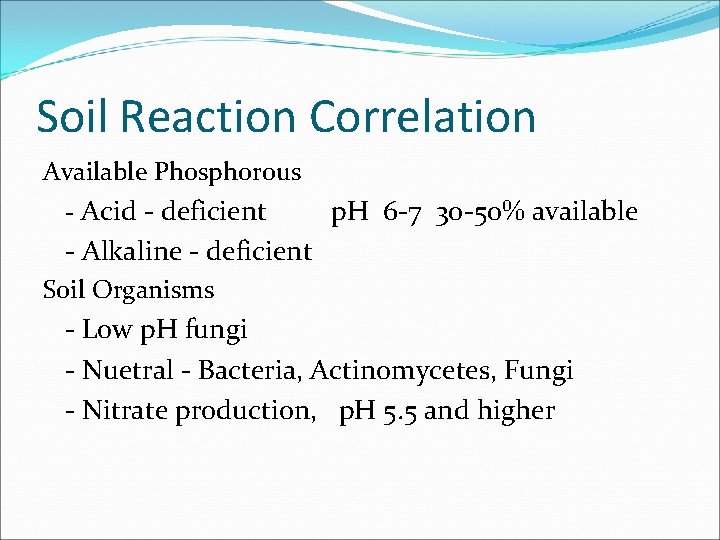
Soil Reaction Correlation Available Phosphorous - Acid - deficient p. H 6 -7 30 -50% available - Alkaline - deficient Soil Organisms - Low p. H fungi - Nuetral - Bacteria, Actinomycetes, Fungi - Nitrate production, p. H 5. 5 and higher

p. H influence on microorganisms Bacteria and actinomicete are reduced at low p. H Nitrification occurs at p. H range 6. 0 to 9. 0, optimum p. H 7 Denitrification (biological loss of N) occurs at a minimu of p. H 5. 5, bellow this point chemical denitrification occurs Nitrogen fixation by Rhyzobium (legume-bacteria symbiosis) optimum occurs between a p. H of 6. 0 to 6. 5 Organic matter decomposition: optimum p. H 7. 0

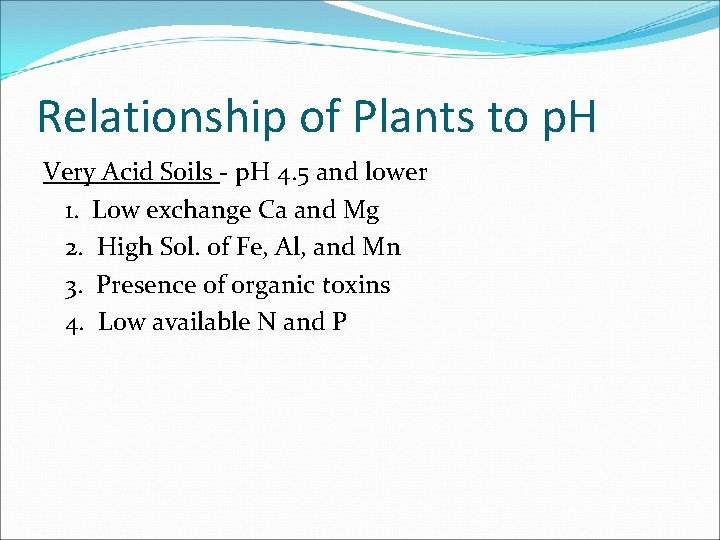
Relationship of Plants to p. H Very Acid Soils - p. H 4. 5 and lower 1. Low exchange Ca and Mg 2. High Sol. of Fe, Al, and Mn 3. Presence of organic toxins 4. Low available N and P

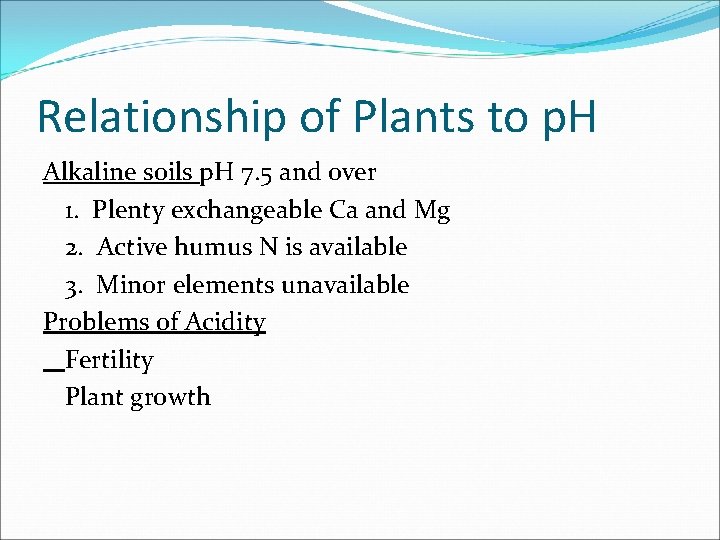
Relationship of Plants to p. H Alkaline soils p. H 7. 5 and over 1. Plenty exchangeable Ca and Mg 2. Active humus N is available 3. Minor elements unavailable Problems of Acidity Fertility Plant growth


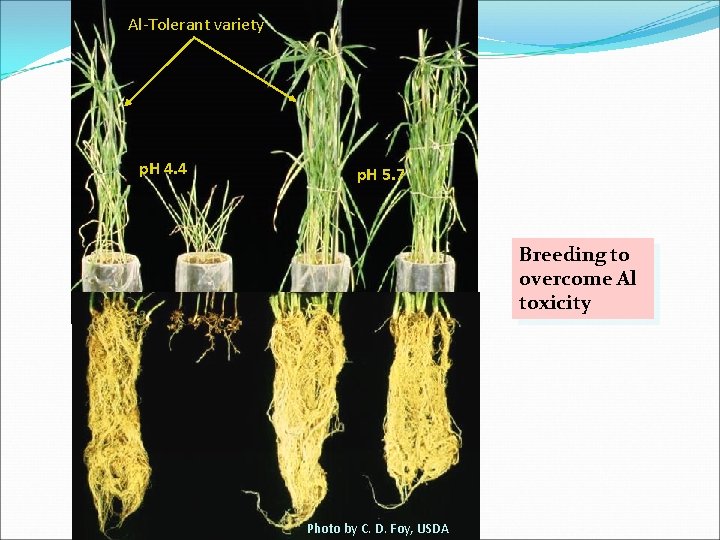
Al-Tolerant variety p. H 4. 4 p. H 5. 7 Breeding to overcome Al toxicity Photo by C. D. Foy, USDA


TERIMA KASIH

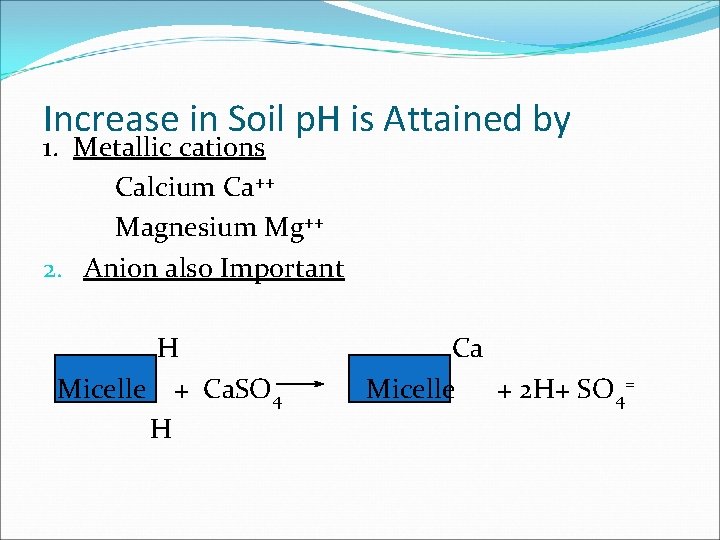
Increase in Soil p. H is Attained by 1. Metallic cations Calcium Ca++ Magnesium Mg++ 2. Anion also Important H Micelle + Ca. SO 4 H Ca Micelle + 2 H+ SO 4=

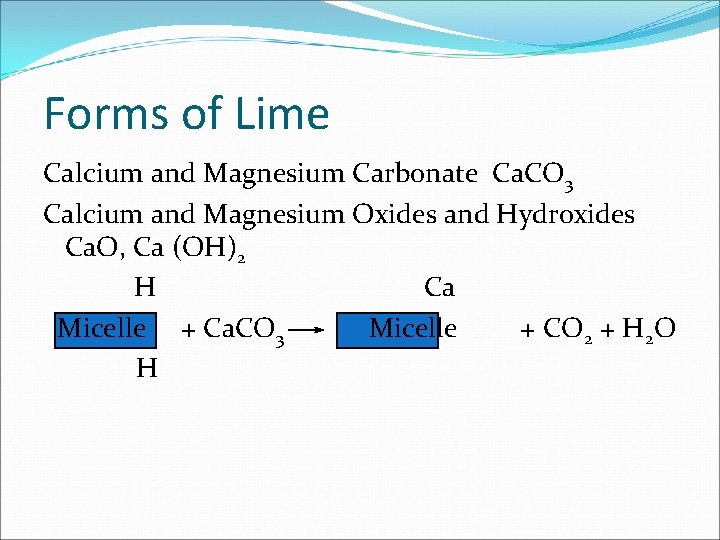
Forms of Lime Calcium and Magnesium Carbonate Ca. CO 3 Calcium and Magnesium Oxides and Hydroxides Ca. O, Ca (OH)2 H Ca Micelle + Ca. CO 3 Micelle + CO 2 + H 2 O H

Why apply lime ? 1. helps nutrients become available to plants 2. improves soil structure 3. provides nutrients for plant growth 4. promotes growth of beneficial microorganisms 5. overcomes acidifying effects of fertilizers 6. reduces metal toxicity to plants (solubility vs. p. H)

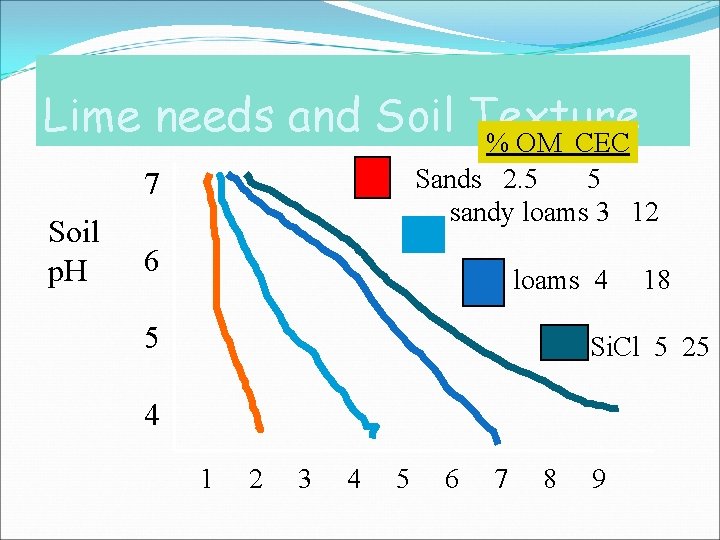
Lime needs and Soil Texture % OM CEC Sands 2. 5 5 sandy loams 3 12 7 Soil p. H 6 loams 4 5 18 Si. Cl 5 25 4 1 2 3 4 5 6 7 8 9

1. Reaction of Saline and Alkali Soils Halomorphic - Salt accumulation in surface horizon a. Saline soils - p. H below 8. 5 (White Alkali) - Na< 15% of CEC - High in Ca, Mg - Leaching won’t change p. H

Reaction of Saline and Alkali Soils b. Saline - alkali soils - Na > 15% of CEC - p. H < 8. 5 - Leaching will increase p. H c. Nonsaline - alkali soils (Black Alkali) - p. H > 8. 5 - Na > 15% of CEC - Low Soluble Salts - Toxic effect of Na+ and OH-

TERIMAKASIH