The Simplest Matter Chapter 1 Section 2 Elements

















- Slides: 31

The Simplest Matter Chapter 1 Section 2

Elements • An element is matter made from one type of atom • At least 115 elements are known and 90 of them occur naturally on Earth. • The other elements are known as synthetic elements and were made in particle accelerators involving nuclear reactions.

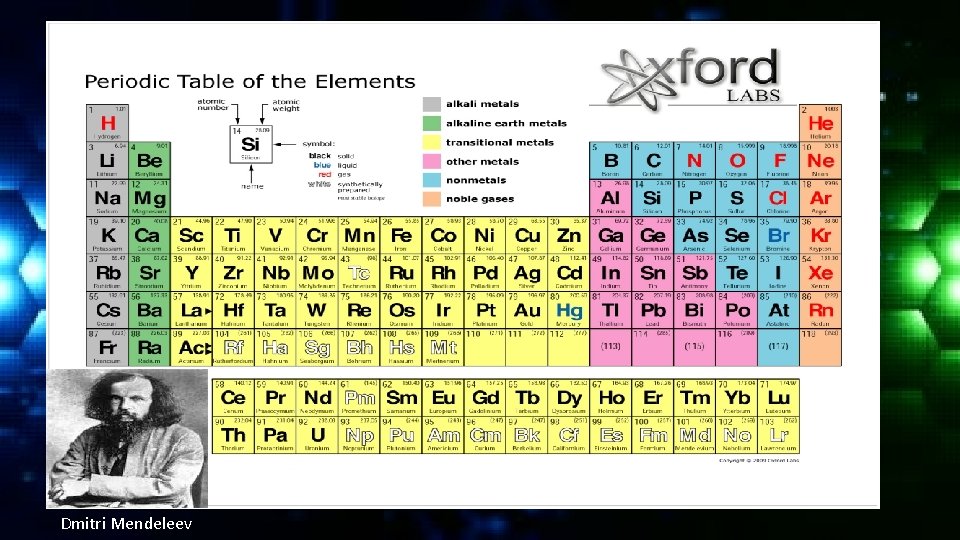
The Periodic Table Dmitri Mendeleev

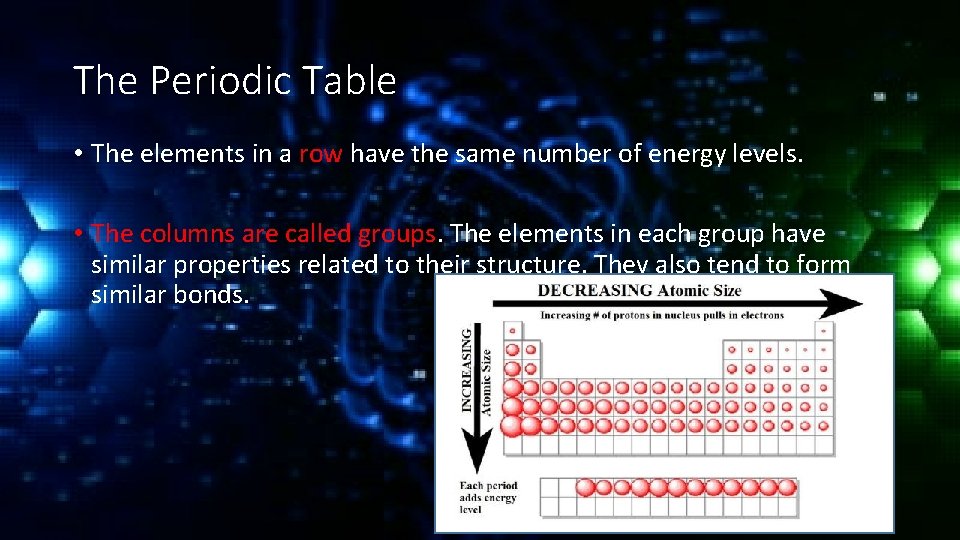
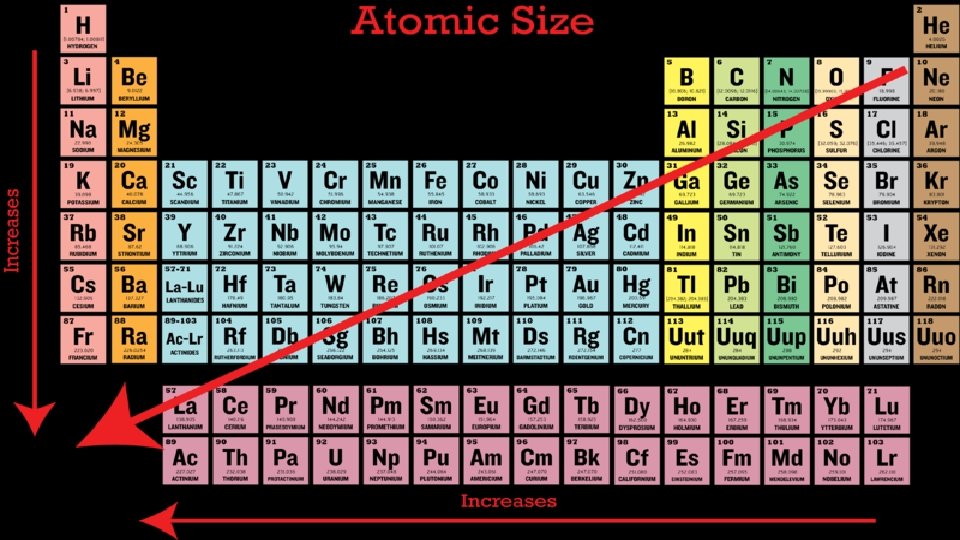
The Periodic Table • Much like the Dewey Decimal system the periodic table is a way of organizing the elements so that information about them is easily accessible. • Each element is represented by a symbol of 1 to 3 letters. This is part of an international system. • The elements are organized based on their relationship to each other. The rows are called periods.

The Periodic Table • The elements in a row have the same number of energy levels. • The columns are called groups. The elements in each group have similar properties related to their structure. They also tend to form similar bonds.

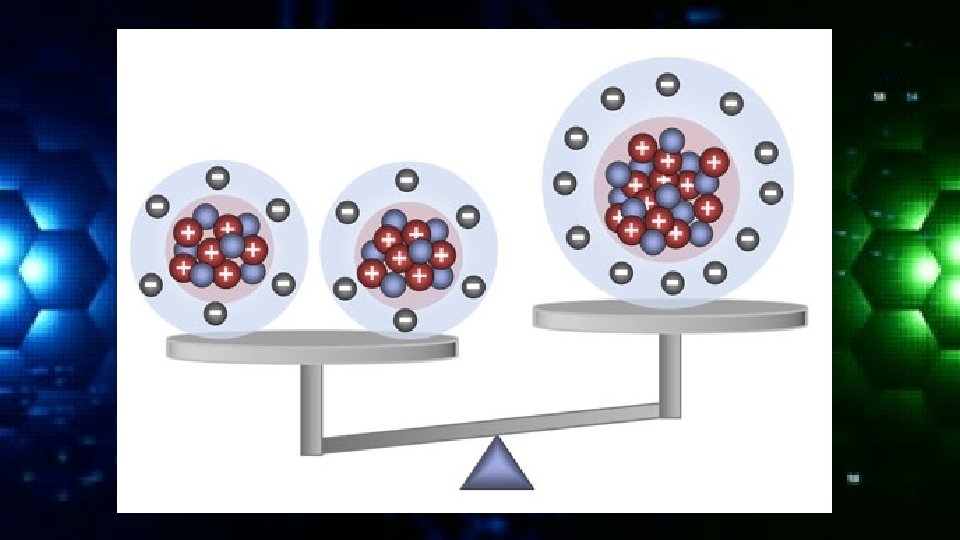
Identifying Characteristics An atom is classified according to the number of its protons and neutrons. The number of protons determines the chemical element The number of neutrons determines the isotope of the element.

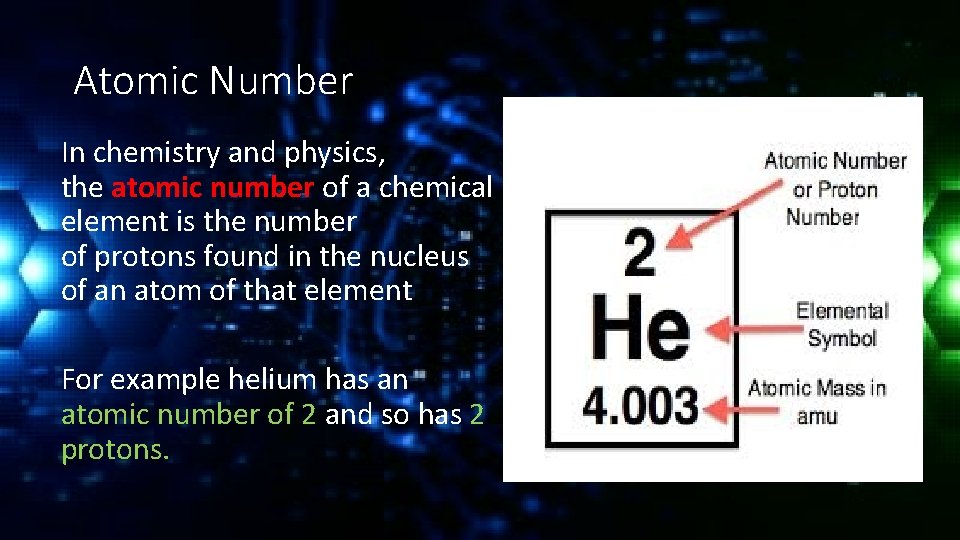
Atomic Number In chemistry and physics, the atomic number of a chemical element is the number of protons found in the nucleus of an atom of that element For example helium has an atomic number of 2 and so has 2 protons.

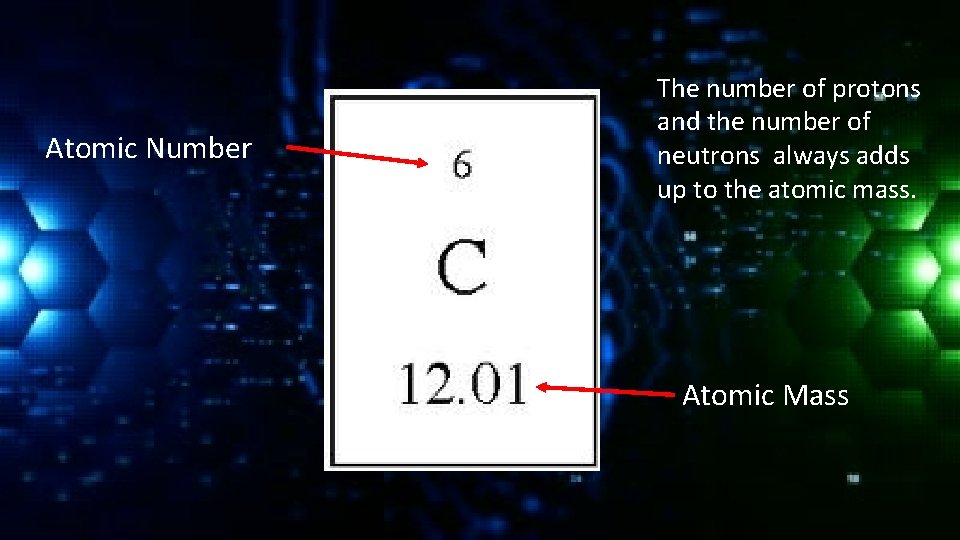
Atomic Mass As you can imagine atoms don’t weigh much. In fact they are so light that we had to invent a new unit to measure them (atomic mass unit – amu) We call this the atomic mass. This measures how many protons and neutrons an atom has. 1 amu is exactly 1/12 the mass of an atom of carbon-12.

Isotopes • Although the number of protons changes from element to element every atom of a particular element has the same number of protons. • The number of neutrons may vary • For example some chlorine atoms have 18 neutrons (chlorine -35) and some have 20 (chlorine-37). They are called isotopes. • You can tell someone exactly which isotope you are referring to by using the mass number.

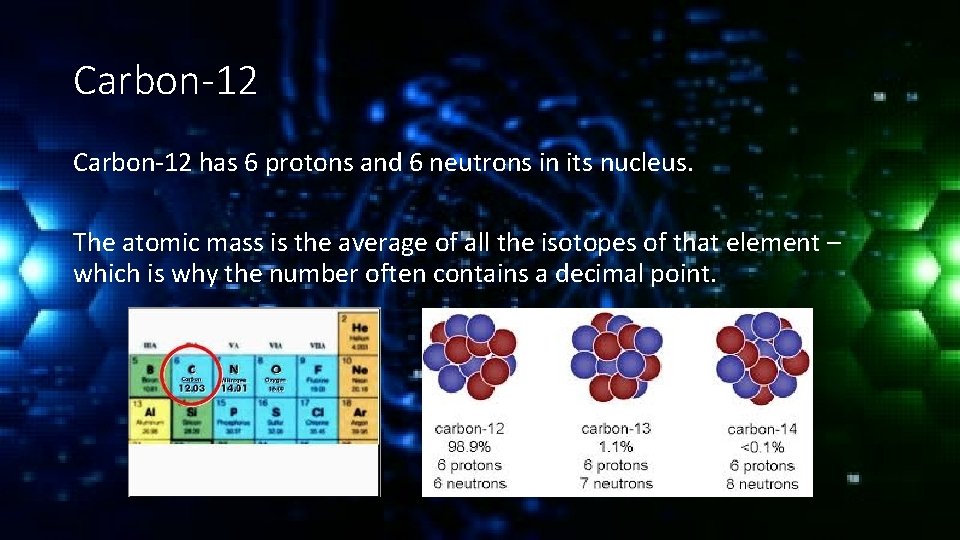
Carbon-12 has 6 protons and 6 neutrons in its nucleus. The atomic mass is the average of all the isotopes of that element – which is why the number often contains a decimal point.

Atomic Number The number of protons and the number of neutrons always adds up to the atomic mass. Atomic Mass

Electrons • Remember the protons carry a positive charge and the neutrons a neutral charge and electrons a negative charge. • The overall charge of an element will be neutral so there must be an equal number of protons and electrons.


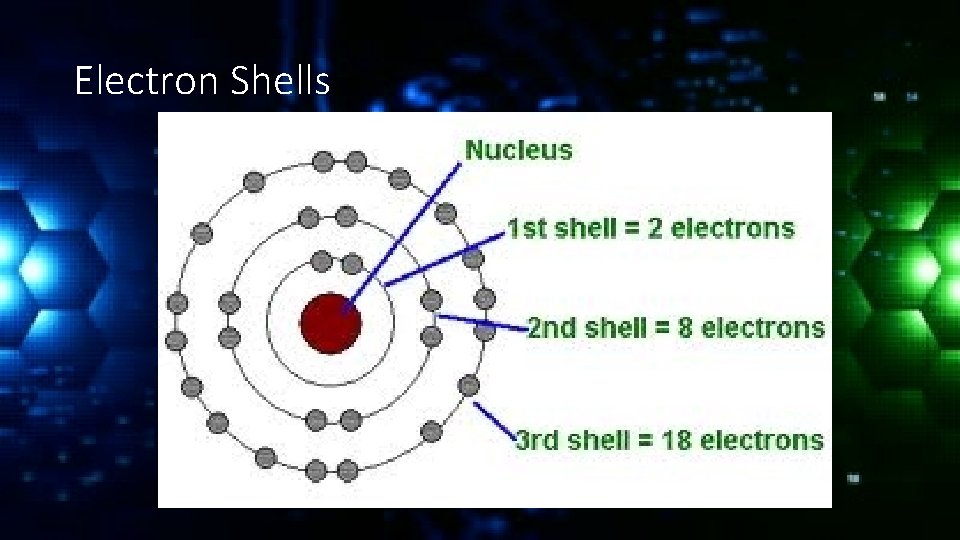
Electron Shells

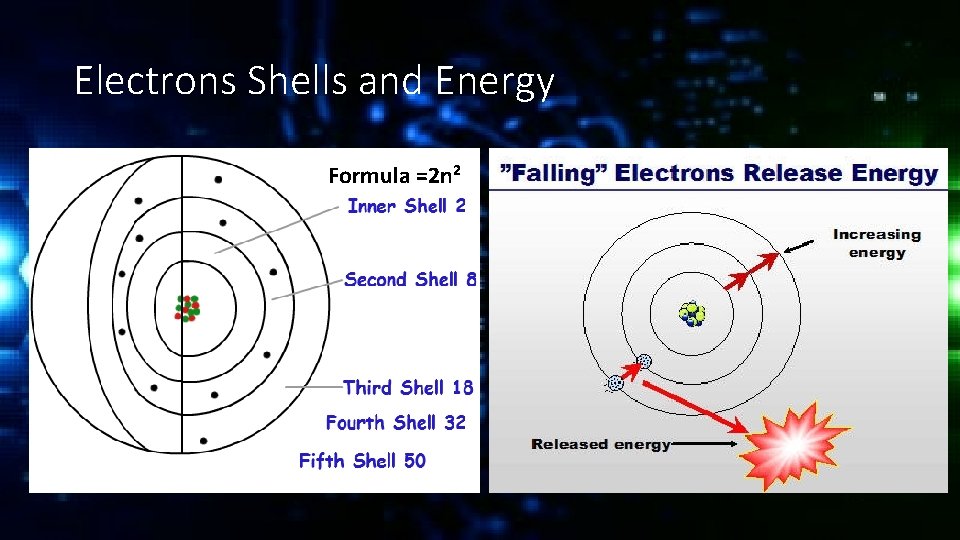
Electrons Shells and Energy Formula =2 n 2

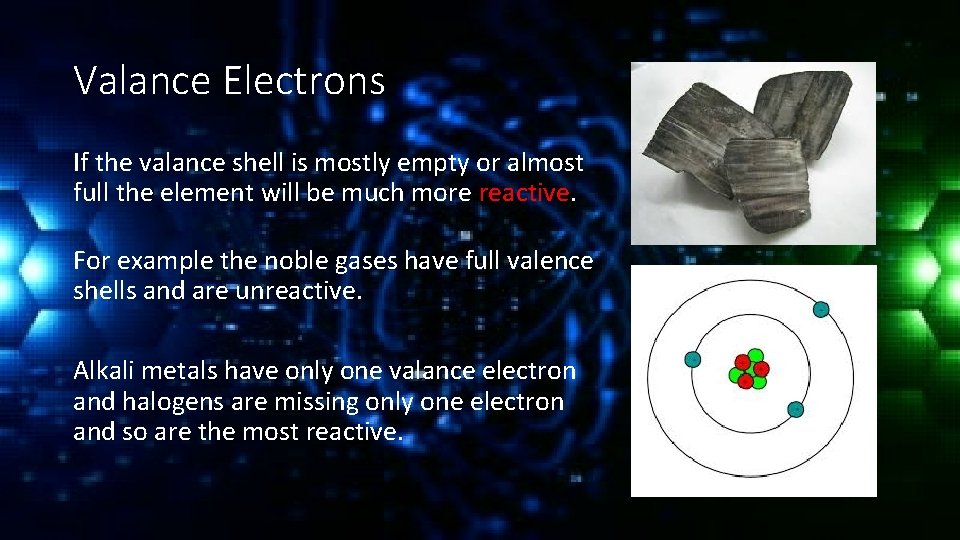
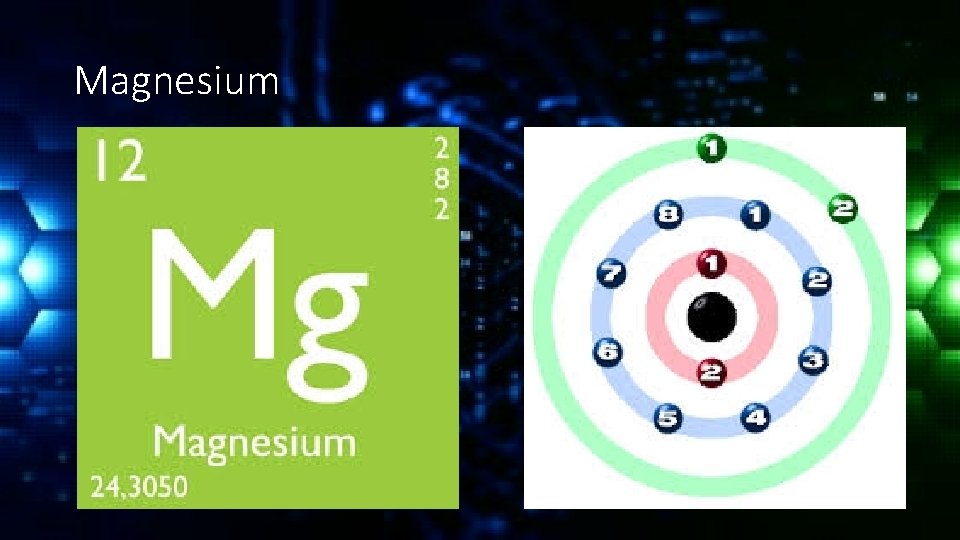
Valance Electrons The electrons in the outermost shell are known as valance electrons and they typically determine how a molecule will react. If the valance shell is full the element will be fairly inert (unreactive).

Valance Electrons If the valance shell is mostly empty or almost full the element will be much more reactive. For example the noble gases have full valence shells and are unreactive. Alkali metals have only one valance electron and halogens are missing only one electron and so are the most reactive.


We are constantly swapping electrons!

Magnesium

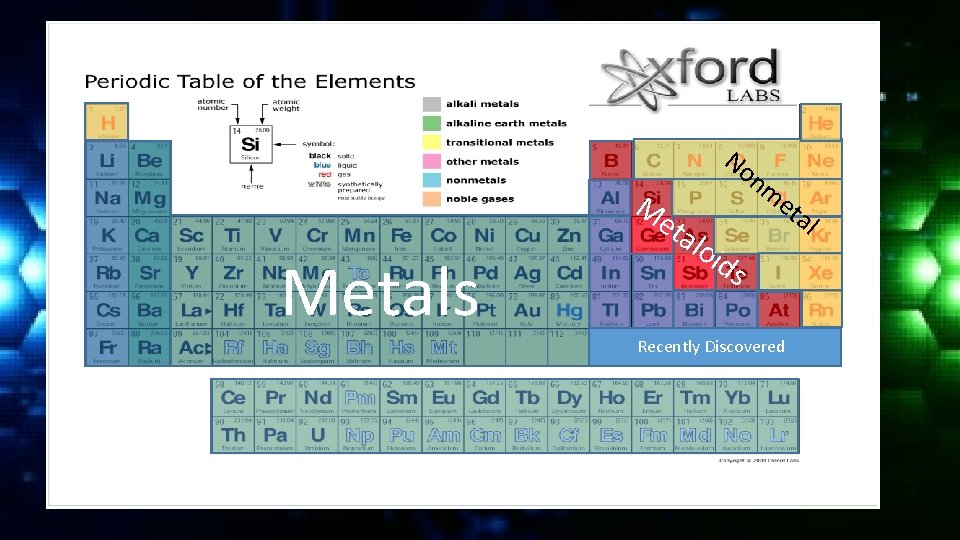
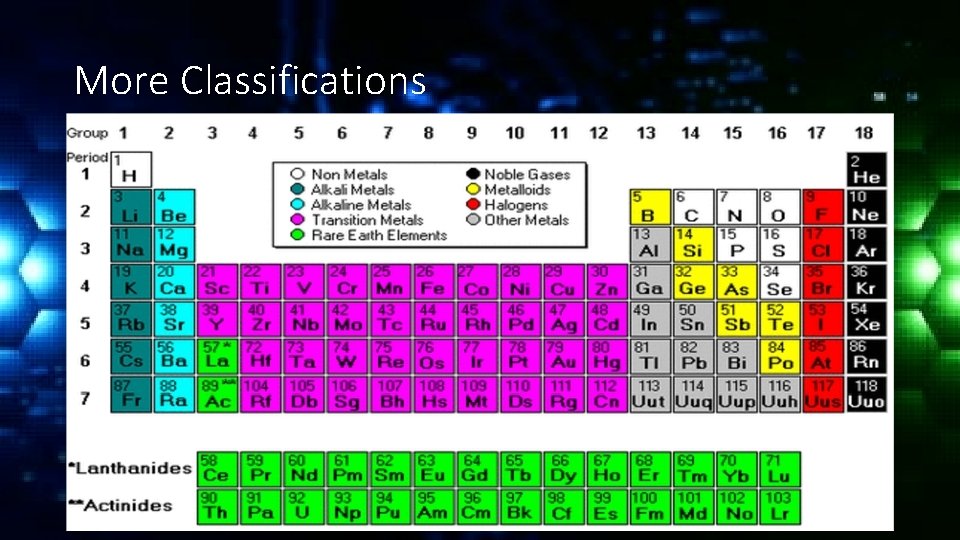
Classification of Elements are classified into 3 general categories; • Metals • Metalloids • Non-metals

Classification of Elements • Metals are usually shiny or metallic in appearance, good conductors of electricity and heat. All metals except mercury are solid at room temperature. Metals are malleable and ductile. Most of the elements are metals.

Classification of Elements • Nonmetals are usually dull in appearance, poor conductors of heat and electricity. Many are gases at room temperature. Solid non-metals are usually brittle. Non-metals are essential for the chemicals of life. • Metalloids have characteristics of metals and nonmetals. All metalloids are solid at room temperature.

The Periodic Table Metals No Me tal nm et oid al s Recently Discovered


More Classifications


Group Work • In your groups go to ptable. com and periodictable. com or use the ipad and find 3 interesting facts about carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur. CHNOPS. • Using the model kits construct a model of each elements atom and draw a diagram of it for your notes. Note: hydrogen atoms are unusual in that they have no neutrons in their nucleus.


Video - Atoms
