The Science of Water 1 Why is water














- Slides: 23

The Science of Water 1

Why is water Essential? • Necessary for life • Water in our atmosphere helps to keep the planet warm. • Our bodies are composed of ~70% dependent on water • Although a person can live without food for more than a month, a person can only live without water for approximately one week. • Your brain is 75 -85% water and plays a vital role in your body's response to dehydration. It controls water intake through altering thirst and varying the water excretion 2 from your kidneys.

Why study water? �The chemical reactions of all living things take place in an aqueous (water based) environment. Thus, water is one of the most important compounds found in living things! 3

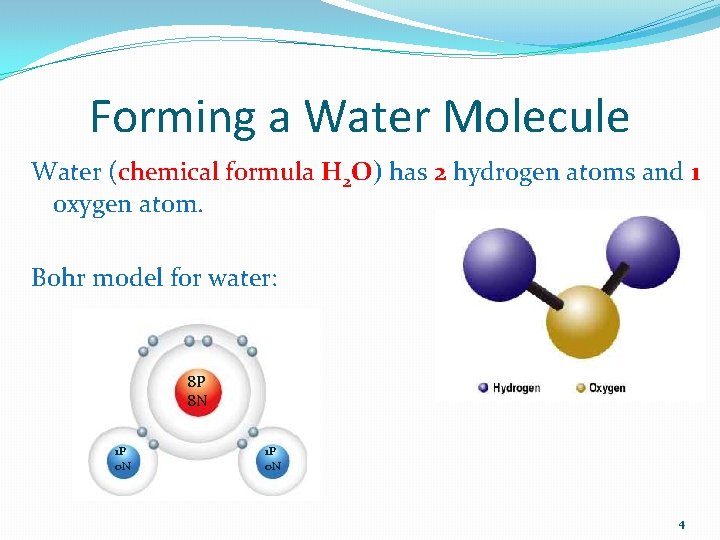
Forming a Water Molecule Water (chemical formula H 2 O) has 2 hydrogen atoms and 1 oxygen atom. Bohr model for water: 8 P 8 N 1 P 0 N 4

Water is POLAR covalent �Recall that electrons are SHARED in a covalent bond. �Polar= electrons are shared unevenly �Oxygen’s 8 p+ attract e- more strongly than Hydrogen’s 1 p+ (more electronegative) �O pulls on e- more electrons more likely to be found near Oxygen) 5

Electronegativity? ! �The attraction of an atom for the electrons of a covalent bond �Tendency for an atom to pull electrons toward itself � 2 atoms of the same element have equal electronegativities � Share electrons equally in a covalent bond, forming a nonpolar covalent bond. 6

Water is POLAR covalent �What does it mean? �Water has a partial + pole & a partial – pole (called dipole) �Acts like a magnet 7

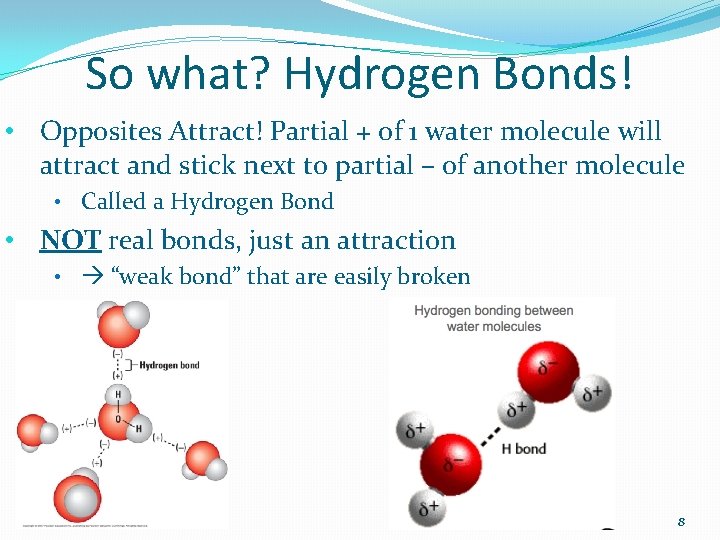
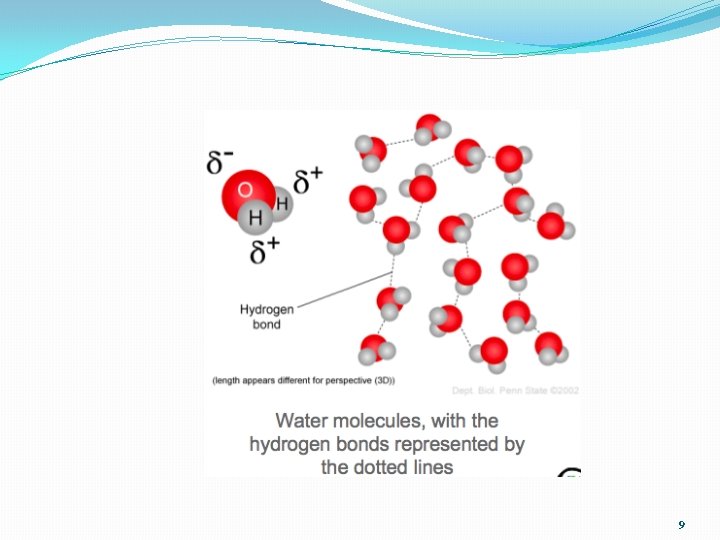
So what? Hydrogen Bonds! • Opposites Attract! Partial + of 1 water molecule will attract and stick next to partial – of another molecule • Called a Hydrogen Bond • NOT real bonds, just an attraction • “weak bond” that are easily broken 8

9

Who Cares? ! �Hydrogen bonds give water its special properties!!! �https: //www. youtube. com/watch? v=3 jw. AGWky 98 c 10

Properties of Water �Adhesion & Cohesion �High Specific Heat �Solid ice is less dense than liquid water �Universal Solvent 11

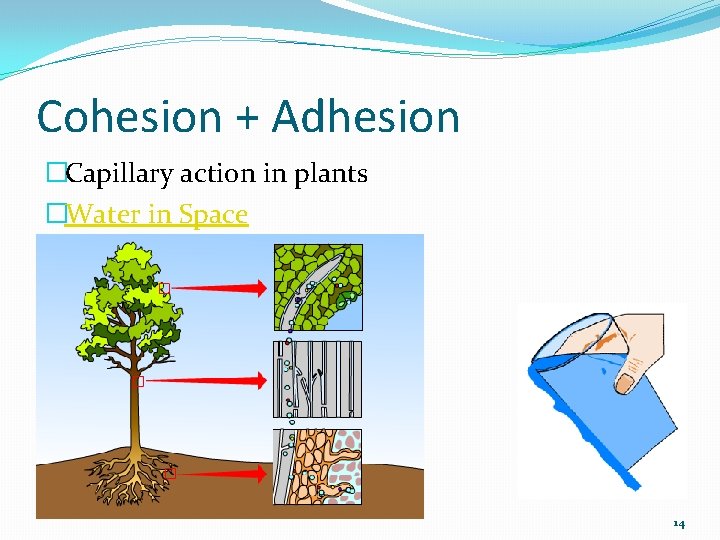
Cohesion �Attraction between molecules of the same substance �Reason water creates beads �Penny experiment! �Responsible for water’s high surface tension �A measure of how difficult it is to stretch or break the surface of a liquid �Water is very cohesive because one water molecule may form as many as 4 hydrogen bonds at the same time. �Jesus Christ Lizard 12

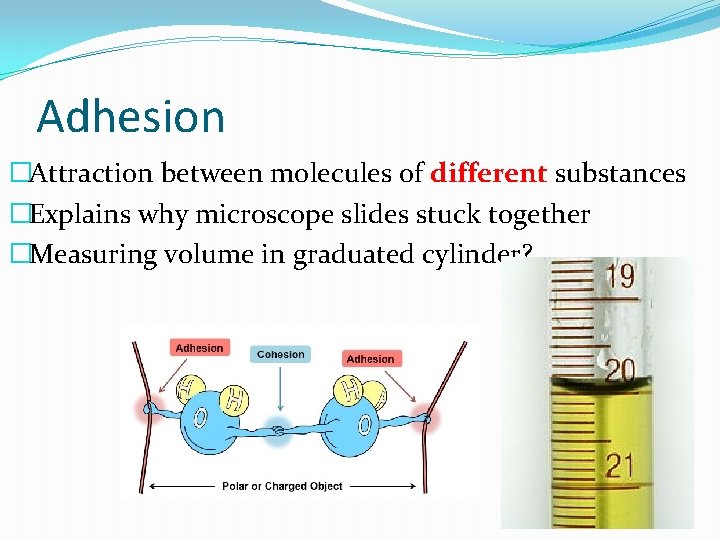
Adhesion �Attraction between molecules of different substances �Explains why microscope slides stuck together �Measuring volume in graduated cylinder? 13

Cohesion + Adhesion �Capillary action in plants �Water in Space 14

High Specific Heat • Specific Heat is the amount of heat energy required to increase the temperature of water. �Water’s multiple hydrogen bonds between its molecules gives it a very high specific heat �Absorbs large amounts of heat energy with only small changes in temperature �Releases heat energy slowly �Moderates the Earth’s climate and helps living organisms regulate their body temperature 15

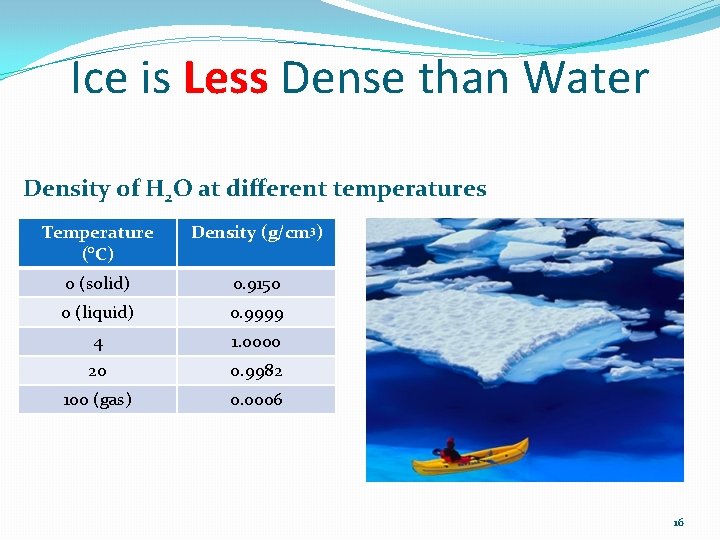
Ice is Less Dense than Water Density of H 2 O at different temperatures Temperature (°C) Density (g/cm 3) 0 (solid) 0. 9150 0 (liquid) 0. 9999 4 1. 0000 20 0. 9982 100 (gas) 0. 0006 16

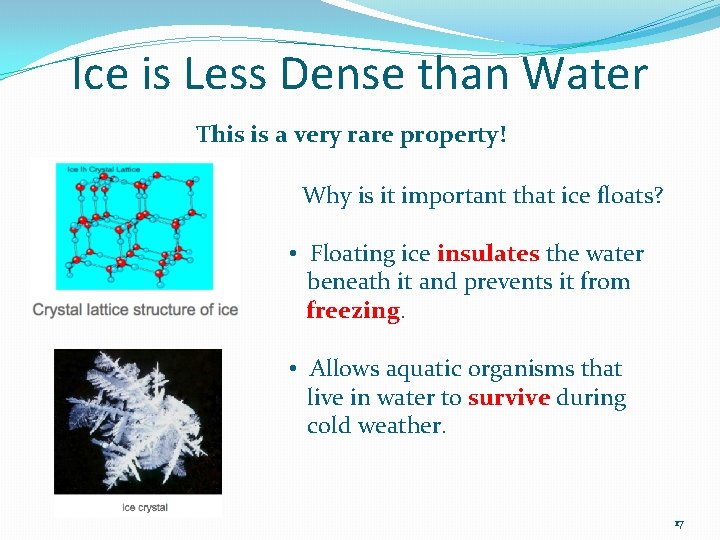
Ice is Less Dense than Water This is a very rare property! Why is it important that ice floats? • Floating ice insulates the water beneath it and prevents it from freezing. • Allows aquatic organisms that live in water to survive during cold weather. 17

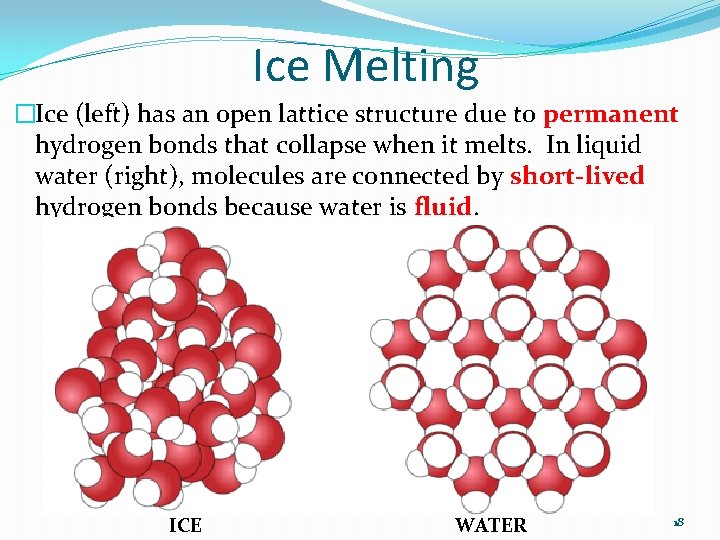
Ice Melting �Ice (left) has an open lattice structure due to permanent hydrogen bonds that collapse when it melts. In liquid water (right), molecules are connected by short-lived hydrogen bonds because water is fluid. ICE WATER 18

Water is a Universal Solvent �The polarity of water allows nearly any polar (unequal distribution of charges) substance to be dissolved in water �Water can dissolve more substances than any other liquid. �Solution = liquid consisting of uniform mixture of two or more substances �Two parts of a solution: � Solvent = liquid (dissolving agent) � Solute = substance dissolved � Example: � Water = solvent Koolaid powder/sugar = solutes Kool-aid = solution 19

Rule to Making Solutions �“Like Dissolves Like” �DO NOT CONFUSE WITH OPPOSITES ATTRACT �Since water is polar, it will dissolve ions and other polar substances �ex: Salt (Na. Cl) breaks down into Na+ and Cl- ions �Hydrophilic = water loving. Dissolves in water. 20

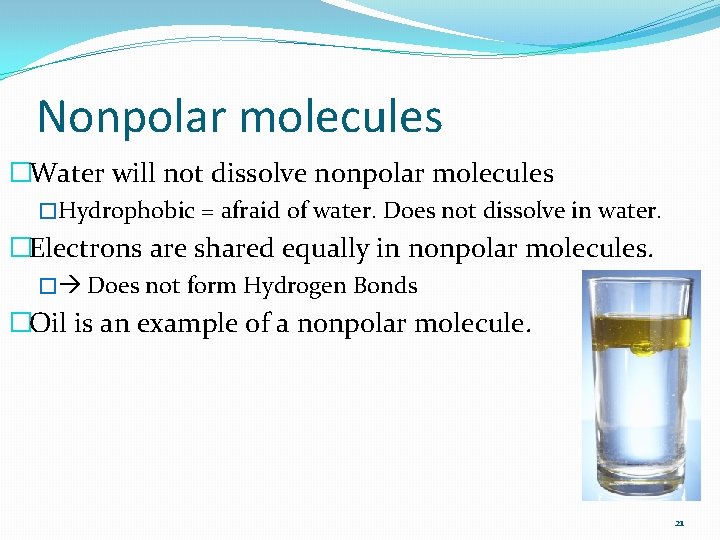
Nonpolar molecules �Water will not dissolve nonpolar molecules �Hydrophobic = afraid of water. Does not dissolve in water. �Electrons are shared equally in nonpolar molecules. � Does not form Hydrogen Bonds �Oil is an example of a nonpolar molecule. 21

Solid, Liquid, and Gas �Water is the only substance which exists under normal conditions on earth as a solid, a liquid, and a gas. 22

High Boiling Point �Water’s high boiling point, allows water to remain as a liquid over most of Earth’s surface �Crucial to life as almost all organisms need liquid water to survive. 23