The Science of Physics Chapter 1 What is












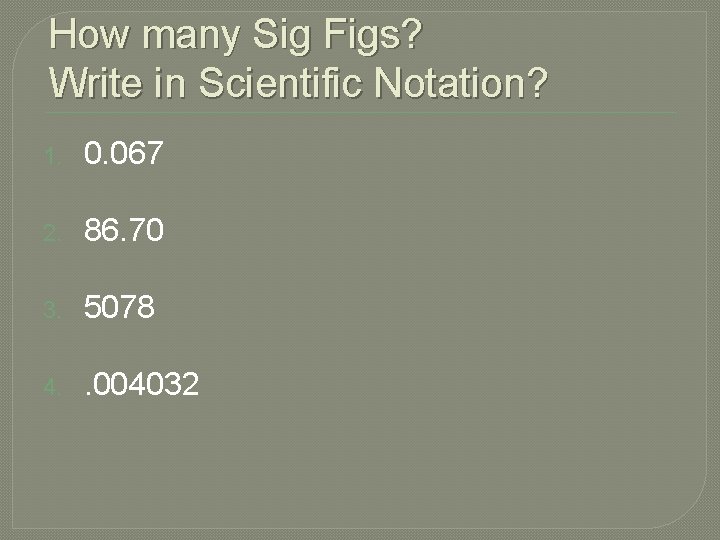



- Slides: 18

The Science of Physics Chapter 1

What is Physics? �Physics – the study of matter, energy, & forces �Purpose: use basic concepts, equations, and assumptions to describe the physical world

Areas within Physics �Mechanics • Motion and its causes • Interactions between objects • Examples: Weight, Friction, Falling/Spinning Bodies �Thermodynamics • Heat and Temperature • Examples: Melting/Freezing, Engines, Refrigerators �Waves/Vibrations • Specific types of repetitive motion • Examples: Springs, Pendulums, Sound

Areas within Physics �Optics • Light • Examples: Mirrors, Lenses, Color, Astronomy �Electromagnetism • Electricity, Magnetism, Light • Examples: Electrical Charge, Permanent Magnets, Electromagnets �Relativity • Movement of Particles at high speeds �Quantum Mechanics • Behavior of submicroscopic particles

Using Models �Model • Diagrams, equations, simulations that describe an object/system/concept • Simplify situations by removing unnecessary information • Help build hypotheses, guide experimental design, and make predictions


Measurements in Experiments

SI Units �Length – distance between 2 objects • Measured in ________ �Mass – amount of matter an object has • Measured in ________ �Time – how long it takes for something to occur • Measured in ________

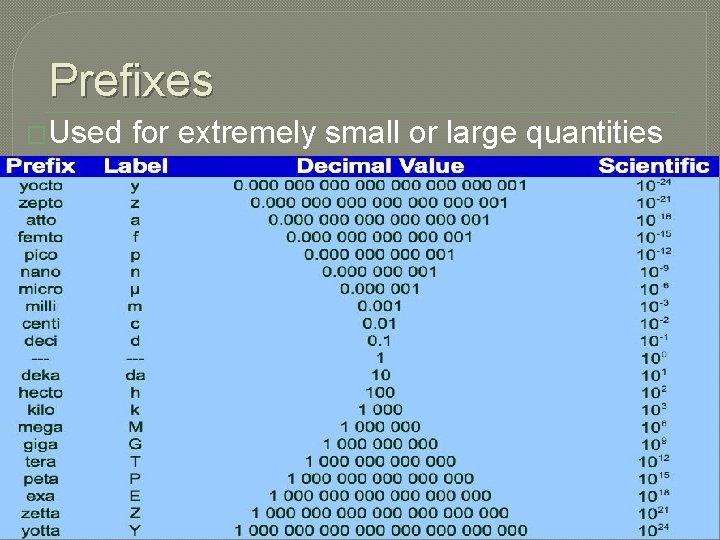
Prefixes �Used for extremely small or large quantities

Unit Conversions �Problem: Convert 3 Kilograms to grams. �Step 1: Set up a Ratio �Step 2: Multiply Original quantity by Ratio �Step 3: Express Answer in Scientific Notation �Step 4: Correctly Label

Unit Conversion Practice 1. 44 x 106 g kg 2. 50 µm m 3. 10 nm mm 4. 1. 5 x 1011 m km

Significant Figures

Significant Figures Rules 1. Zeros between other Non-zero digits ARE 3. 0025 2. Zeros in front of nonzero digits are NOT. 0008 3. Zeros at the end of a number and also to the right of the decimal ARE 57. 00 4. Zeros at the end of a number and to the left of a decimal are NOT 1000 m
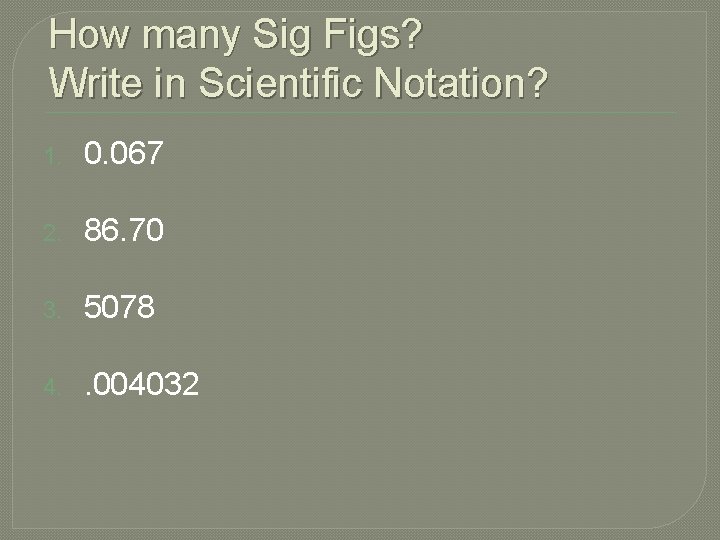
How many Sig Figs? Write in Scientific Notation? 1. 0. 067 2. 86. 70 3. 5078 4. . 004032

Calculating With Sig Figs �Adding/Subtracting • Round final answer to first column from the left containing an estimated digit 97. 3 + 5. 85 �Multiplying/Dividing • Final answer has same number of sig figs as value with the smallest number of sig figs 123 X 5. 35

Accuracy & Precision �Accuracy – how close a measurement is to the correct value �Precision – how exact a measurement is 1. 325 m vs 1. 3 m

Calculating Accuracy/Precision The density of lead is 11. 34 g/cm 3 Subject A measured 11. 32 g/cm 3 11. 35 g/cm 3 Subject B measured 11. 43 g/cm 3 11. 44 g/cm 3 11. 33 g/cm 3 11. 42 g/cm 3

Chapter 1 Review �Page 30