The s p d and f blocks Number
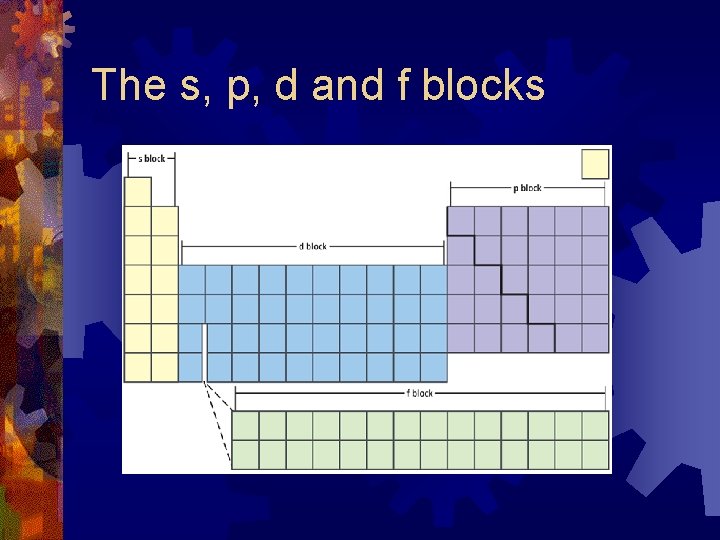
The s, p, d and f blocks

Number of Valence Electrons Elements on the right • Nonmetals • 4 or more valence electron • tend to gain electrons • become negative ions Elements on the left • Metals • 3 or less valence electrons • tend to lose valence electrons • form positive ions
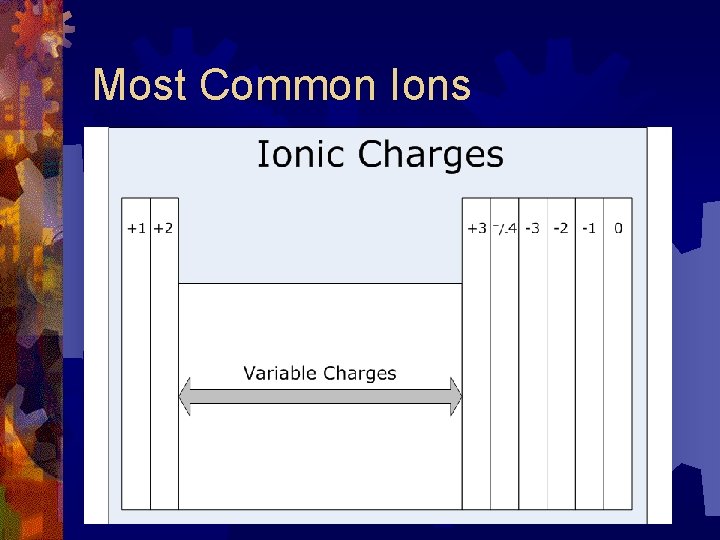
Most Common Ions

The Octet Rule ® Atoms tend to gain, lose or share electrons in order to acquire a full set of eight valence electrons. ® Elements on the left (metals) tend to lose valence electrons and form positive ions ® Elements on the right (nonmetals) tend to gain electrons to become negative ions

Trends ® Atomic Radius ® Ionization Energy ® Electronegativity
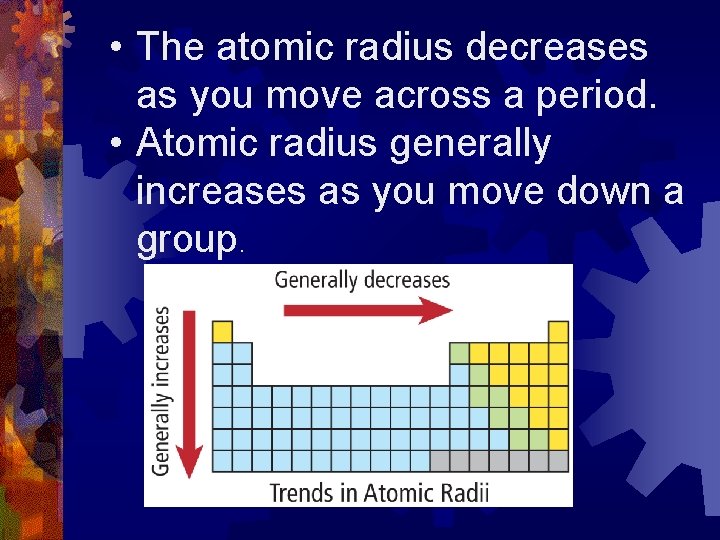
• The atomic radius decreases as you move across a period. • Atomic radius generally increases as you move down a group.
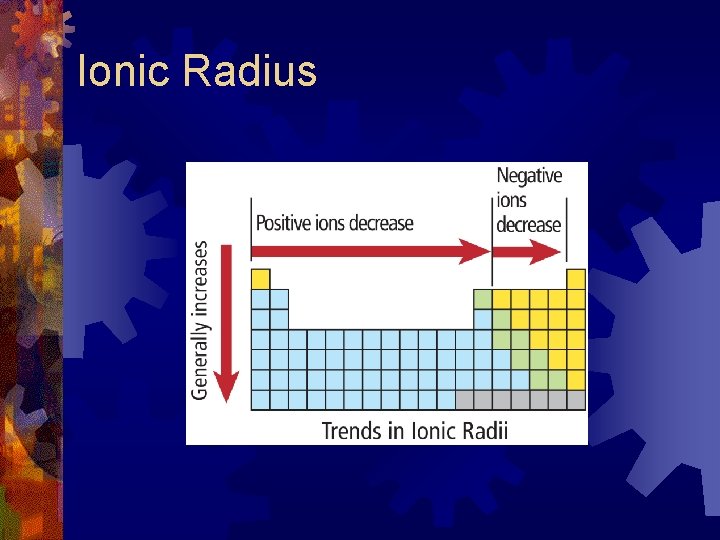
Ionic Radius

Ionization Energy ® the amount of energy need to remove an electron from a specific atom or ion in its ground state in the gas phase ® High Ionization Energy: atom is holding onto electrons very strongly ® Low Ionization Energy: atom is holding electrons less tightly
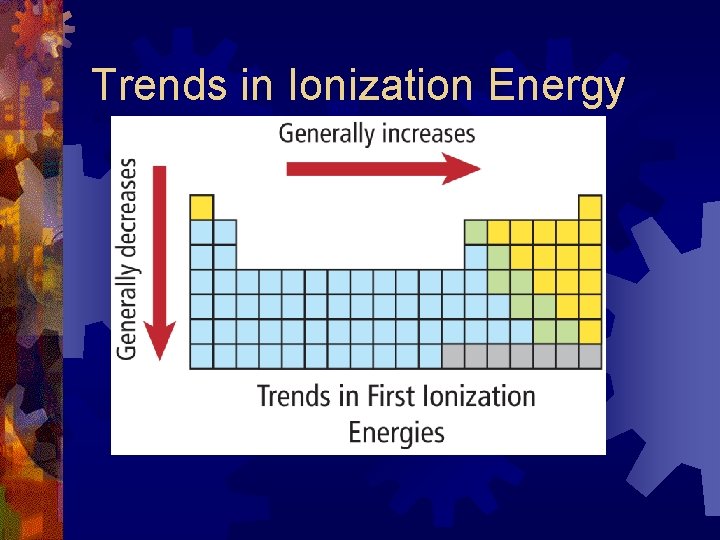
Trends in Ionization Energy

Electronegativity ® The ability of an an atom to attract electrons to itself when it is combined with another atom ® Expressed in terms of a relative scale: fluorine is assigned a value of 4 and all other elements are calculated relative to this. ® The units of electronegativity are arbitrary units called Paulings. ® Noble gases have no values because of few chemical compounds
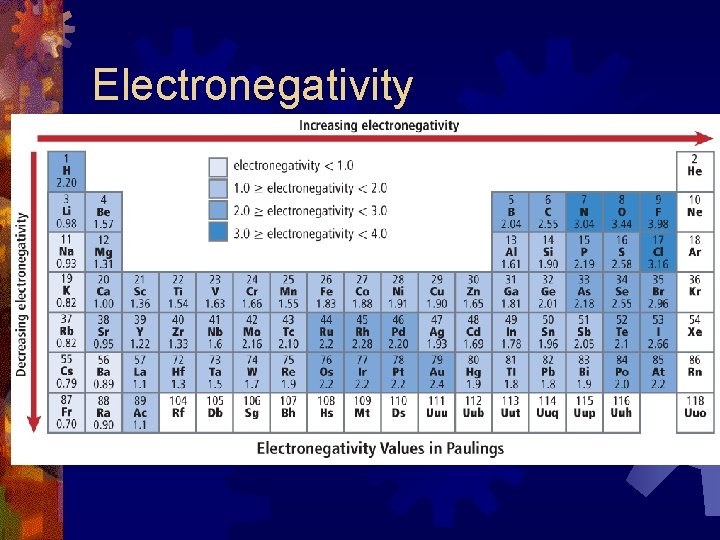
Electronegativity
- Slides: 11