The Role of Proteasome Inhibition in Multiple Myeloma

The Role of Proteasome Inhibition in Multiple Myeloma SC-ch-CARFILZOMI-01015 -00154
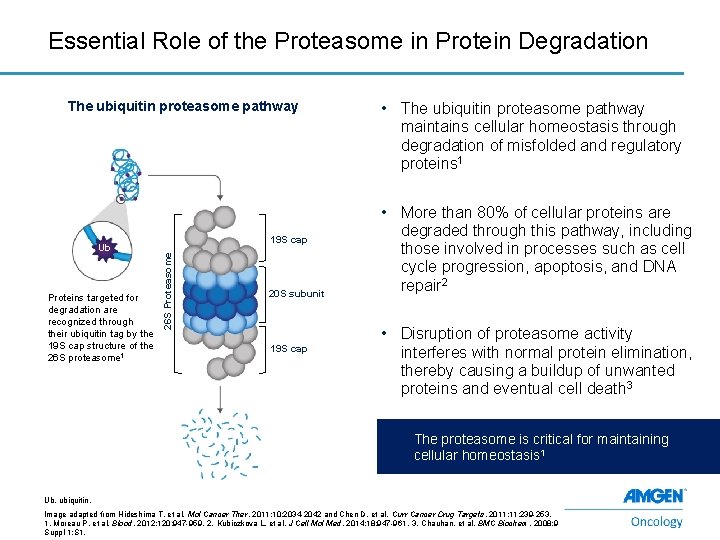
Essential Role of the Proteasome in Protein Degradation The ubiquitin proteasome pathway Proteins targeted for degradation are recognized through their ubiquitin tag by the 19 S cap structure of the 26 S proteasome 1 19 S cap 26 S Proteasome Ub 20 S subunit 19 S cap • The ubiquitin proteasome pathway maintains cellular homeostasis through degradation of misfolded and regulatory proteins 1 • More than 80% of cellular proteins are degraded through this pathway, including those involved in processes such as cell cycle progression, apoptosis, and DNA repair 2 • Disruption of proteasome activity interferes with normal protein elimination, thereby causing a buildup of unwanted proteins and eventual cell death 3 The proteasome is critical for maintaining cellular homeostasis 1 Ub, ubiquitin. 2 Image adapted from Hideshima T, et al. Mol Cancer Ther. 2011; 10: 2034 -2042 and Chen D, et al. Curr Cancer Drug Targets. 2011; 11: 239 -253. 1. Moreau P, et al. Blood. 2012; 120: 947 -959. 2. Kubiczkova L, et al. J Cell Mol Med. 2014; 18: 947 -961. 3. Chauhan, et al. BMC Biochem. 2008; 9 Suppl 1: S 1.
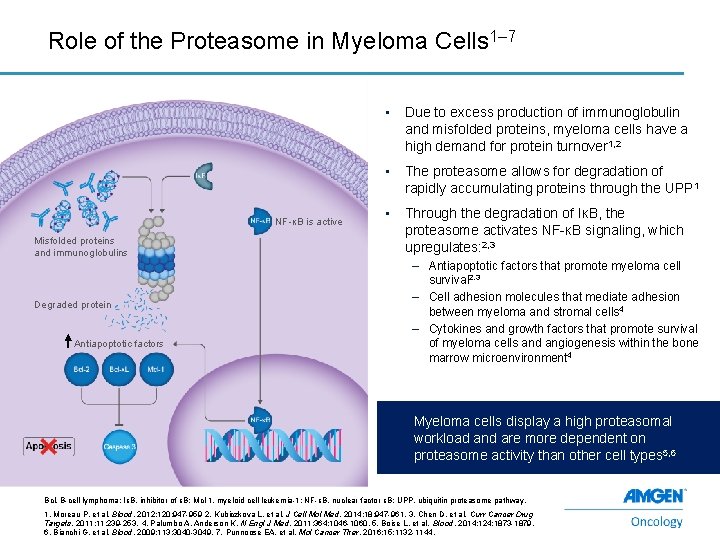
Role of the Proteasome in Myeloma Cells 1– 7 NF-κB is active Misfolded proteins and immunoglobulins Degraded protein Antiapoptotic factors • Due to excess production of immunoglobulin and misfolded proteins, myeloma cells have a high demand for protein turnover 1, 2 • The proteasome allows for degradation of rapidly accumulating proteins through the UPP 1 • Through the degradation of IκB, the proteasome activates NF-κB signaling, which upregulates: 2, 3 – Antiapoptotic factors that promote myeloma cell survival 2, 3 – Cell adhesion molecules that mediate adhesion between myeloma and stromal cells 4 – Cytokines and growth factors that promote survival of myeloma cells and angiogenesis within the bone marrow microenvironment 4 Myeloma cells display a high proteasomal workload and are more dependent on proteasome activity than other cell types 5, 6 Bcl, B-cell lymphoma; IκB, inhibitor of κB; Mcl-1, myeloid cell leukemia-1; NF-κB, nuclear factor κB; UPP, ubiquitin proteasome pathway. 3 1. Moreau P, et al. Blood. 2012; 120: 947 -959 2. Kubiczkova L, et al. J Cell Mol Med. 2014; 18: 947 -961. 3. Chen D, et al. Curr Cancer Drug Targets. 2011; 11: 239 -253. 4. Palumbo A, Anderson K. N Engl J Med. 2011; 364: 1046 -1060. 5. Boise L, et al. Blood. 2014; 124: 1873 -1879. 6. Bianchi G, et al. Blood. 2009; 113: 3040 -3049. 7. Punnoose EA, et al. Mol Cancer Ther. 2016; 15: 1132 -1144.

Impact of Proteasome Inhibitors in Myeloma Cells Proteasome inhibitors target myeloma cell biology and microenvironment • Within the myeloma cell: Misfolded proteins and immunoglobulins IκB is not degraded and binds NF-κB is inactive – Accumulation of misfolded and regulatory proteins triggers endoplasmic reticulum (ER) stress, which activates the unfolded protein response (UPR) and leads to apoptosis 1, 2 – Proteasome inhibition suppresses the NF-κB pathway, downregulating antiapoptotic factors 1, 3 • In the bone marrow microenvironment: – Proteasome inhibition downregulates cytokine secretion and cell proliferation, adhesion, and migration and decreases tumor angiogenesis 4 Antiapoptotic factors No activation by NF-κB Proteasome inhibitors disrupt the normal removal of intracellular proteins, causing myeloma cells to undergo apoptosis 1, 2 Bcl, B-cell lymphoma; ER, endoplasmic reticulum; I κB, inhibitor of κB; Mcl-1, myeloid cell leukemia-1; NF-κB, nuclear factor κB; UPR, unfolded protein response. 4 1. Meister S, et al. Cancer Res. 2007; 67: 1783 -1792. 2. Moreau P, et al. Blood. 2012; 120: 947 -959 3. Chen D, et al. Curr Cancer Drug Targets. 2011; 11: 239 -253. 4. Palumbo A, Anderson K. N Engl J Med. 2011; 364: 1046 -1060.

Proteasome Inhibitors Target Sites Within the Catalytic Core • Proteasome inhibitors (PIs) target both the constitutive proteasome and the immunoproteasome in myeloma cells. The immunoproteasome is an alternative proteasome isoform that can be formed in response to inflammatory conditions 1– 3 Constitutive proteasome 1, 2 PI binding site Immunoproteasome 1, 2 b 7 β 1 β 6 C-L β 2 CT-L β 4 C-L β b ring β 5 LMP 2 β 6 α β α MECL 1 LMP 7 CT-L b 3 T-L β 4 β 3 • Proteolytic cleavage is mediated by chymotrypsin-like (CT-L), trypsin-like (T-L), and caspase-like (C-L) sites within the 20 S core of the constitutive proteasome and the immunoproteasome 2 – CT-L activity is the rate-limiting step for proteolysis and is therefore a critical target for PIs 4 PIs target sites within the proteasome’s catalytic core to inhibit proteasome activity and promote myeloma cell death 2 C-L, caspase-like; CT-L, chymotrypsin-like; LMP, low-molecular mass polypeptide; MECL 1, multicatalytic endopeptidase complex-like; PI, proteasome inhibitor; T-L, trypsin-like. 5 Image Adapted from Groeuttrup M, et al. Nat Rev Immunol. 2010; 10: 73 -78. 1. Groeuttrup M, et al. Nat Rev Immunol. 2010; 10: 73 -78. 2. Moreau P, et al. Blood. 2012; 120: 947 -959. 3. Kuhn D, et al. Blood. 2007; 110: 3281 -3290. 4. Khan M, Stewart A. Future Oncol. 2011; 7: 607 -612.
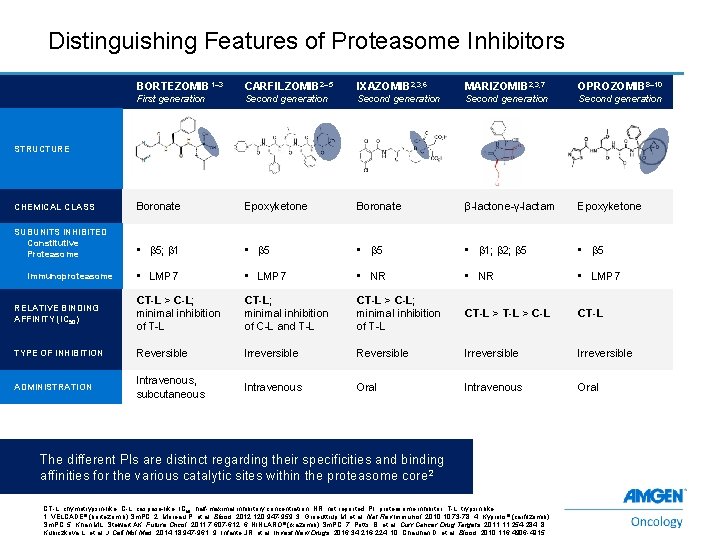
Distinguishing Features of Proteasome Inhibitors BORTEZOMIB 1– 3 CARFILZOMIB 2– 5 IXAZOMIB 2, 3, 6 MARIZOMIB 2, 3, 7 OPROZOMIB 8– 10 First generation Second generation CHEMICAL CLASS Boronate Epoxyketone Boronate β-lactone-γ-lactam Epoxyketone SUBUNITS INHIBITED Constitutive Proteasome • b 5; b 1 • b 5 • b 1; b 2; b 5 • LMP 7 • NR • LMP 7 RELATIVE BINDING AFFINITY (IC 50) CT-L > C-L; minimal inhibition of T-L CT-L; minimal inhibition of C-L and T-L CT-L > C-L; minimal inhibition of T-L CT-L > C-L CT-L TYPE OF INHIBITION Reversible Irreversible ADMINISTRATION Intravenous, subcutaneous Intravenous Oral STRUCTURE Immunoproteasome The different PIs are distinct regarding their specificities and binding affinities for the various catalytic sites within the proteasome core 2 6 CT-L, chymotrypsin-like; C-L, caspase-like; IC 50, half-maximal inhibitory concentration; NR, not reported; PI, proteasome inhibitor; T-L, trypsin-like. 1. VELCADE® (bortezomib) Sm. PC. 2. Moreau P, et al. Blood. 2012; 120: 947 -959. 3. Groeuttrup M, et al. Nat Rev Immunol. 2010; 10: 73 -78. 4. Kyprolis® (carfilzomib) Sm. PC. 5. Khan ML, Stewart AK. Future Oncol. 2011; 7: 607 -612. 6. NINLARO ® (ixazomib) Sm. PC. 7. Potts, B, et al. Curr Cancer Drug Targets. 2011; 11: 254 -284. 8. Kubiczkova L, et al. J Cell Mol Med. 2014; 18: 947 -961. 9. Infante JR, et al. Invest New Drugs. 2016; 34: 216 -224. 10. Chauhan D, et al. Blood. 2010; 116: 4906 -4915.
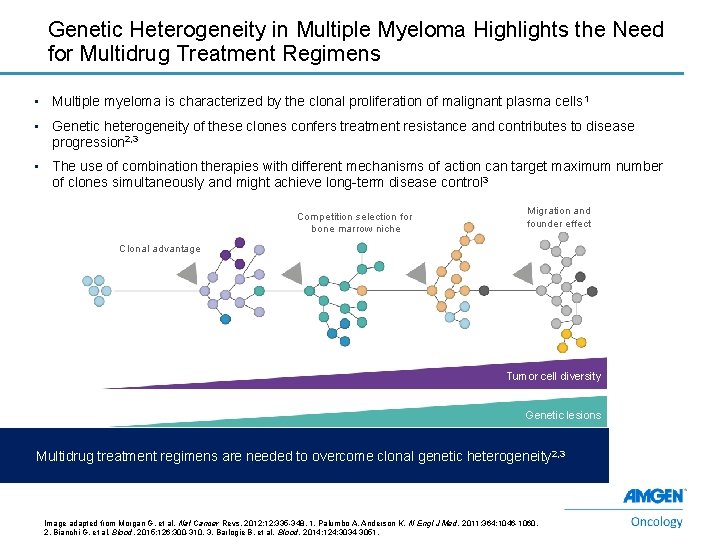
Genetic Heterogeneity in Multiple Myeloma Highlights the Need for Multidrug Treatment Regimens • Multiple myeloma is characterized by the clonal proliferation of malignant plasma cells 1 • Genetic heterogeneity of these clones confers treatment resistance and contributes to disease progression 2, 3 • The use of combination therapies with different mechanisms of action can target maximum number of clones simultaneously and might achieve long-term disease control 3 Competition selection for bone marrow niche Migration and founder effect Clonal advantage Tumor cell diversity Genetic lesions Multidrug treatment regimens are needed to overcome clonal genetic heterogeneity 2, 3 7 Image adapted from Morgan G, et al. Nat Cancer Revs. 2012; 12: 335 -348. 1. Palumbo A, Anderson K. N Engl J Med. 2011; 364: 1046 -1060. 2. Bianchi G, et al. Blood. 2015; 126: 300 -310. 3. Barlogie B, et al. Blood. 2014; 124: 3034 -3051.
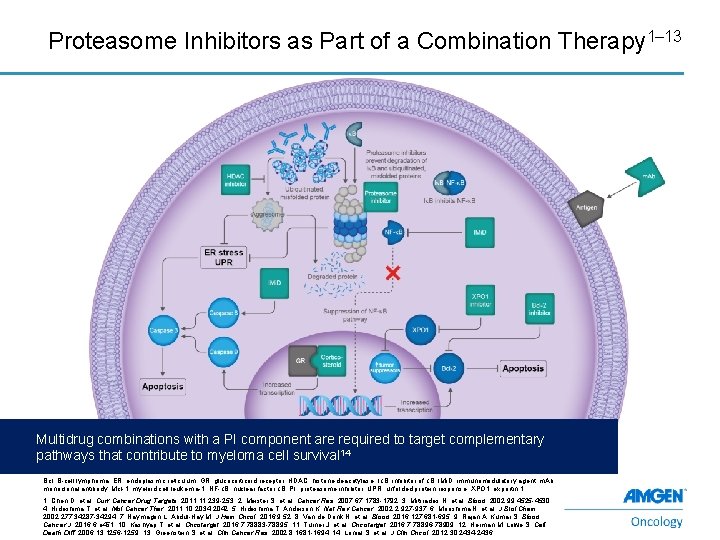
Proteasome Inhibitors as Part of a Combination Therapy 1– 13 Multidrug combinations with a PI component are required to target complementary pathways that contribute to myeloma cell survival 14 Bcl, B-cell lymphoma; ER, endoplasmic reticulum, GR, glucocorticoid receptor; HDAC, histone deacetylase; IκB, inhibitor of κB; IMi. D, immunomodulatory agent; m. Ab, monoclonal antibody; Mcl-1, myeloid cell leukemia-1; NF-κB, nuclear factor κB; PI, proteasome inhibitor; UPR, unfolded protein response; XPO 1, exportin 1. 8 1. Chen D, et al. Curr Cancer Drug Targets. 2011; 11: 239 -253. 2. Meister S, et al. Cancer Res. 2007; 67: 1783 -1792. 3. Mitsiades N, et al. Blood. 2002; 99: 4525 -4530. 4. Hideshima T, et al. Mol Cancer Ther. 2011; 10: 2034 -2042. 5. Hideshima T, Anderson K. Nat Rev Cancer. 2002; 2: 927 -937. 6. Morishima N, et al. J Biol Chem. 2002; 277: 34287 -34294. 7. Naymagon L, Abdul-Hay M. J Hem Oncol. 2016; 9: 52. 8. Van de Donk N, et al. Blood. 2016; 127: 681 -695. 9. Rajan A, Kumar S. Blood Cancer J. 2016; 6: e 451. 10. Kashyap T, et al. Oncotarget. 2016; 7: 78883 -78895. 11. Turner J, et al. Oncotarget. 2016; 7: 78896 -78909. 12. Herman M, Lowe S. Cell Death Diff. 2006; 13: 1256 -1259. 13. Greenstein S, et al. Clin Cancer Res. 2002; 8: 1681 -1694. 14. Lonial S, et al. J Clin Oncol. 2012; 30: 2434 -2436.
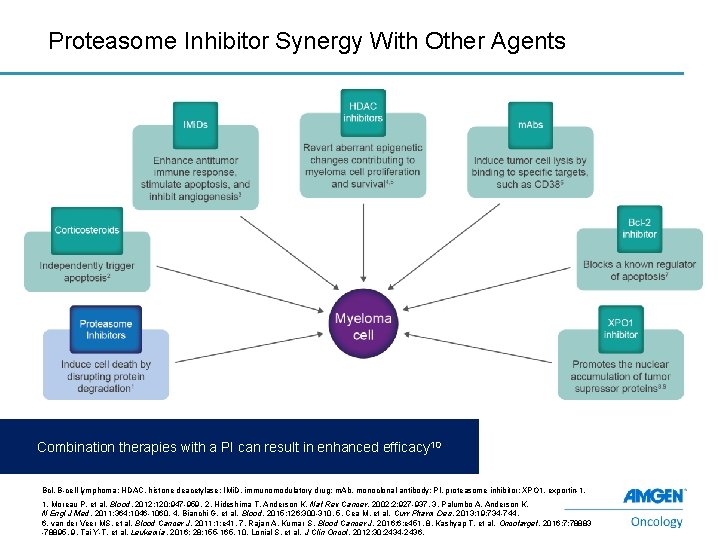
Proteasome Inhibitor Synergy With Other Agents Combination therapies with a PI can result in enhanced efficacy 10 Bcl, B-cell lymphoma; HDAC, histone deacetylase; IMi. D, immunomodulatory drug; m. Ab, monoclonal antibody; PI, proteasome inhibitor; XPO 1, exportin-1. 9 1. Moreau P, et al. Blood. 2012; 120: 947 -959. 2. Hideshima T, Anderson K. Nat Rev Cancer. 2002; 2: 927 -937. 3. Palumbo A, Anderson K. N Engl J Med. 2011; 364: 1046 -1060. 4. Bianchi G, et al. Blood. 2015; 126: 300 -310. 5. Cea M, et al. Curr Pharm Des. 2013; 19: 734 -744. 6. van der Veer MS, et al. Blood Cancer J. 2011; 1: e 41. 7. Rajan A, Kumar S. Blood Cancer J. 2016; 6: e 451. 8. Kashyap T, et al. Oncotarget. 2016; 7: 78883 -78895. 9. Tai Y-T, et al. Leukemia. 2016; 28: 155 -165. 10. Lonial S, et al. J Clin Oncol. 2012; 30: 2434 -2436.

Proteasome Inhibitors and Corticosteroids • Corticosteroids trigger myeloma cell death, which is associated with: – Activation of proapoptotic related adhesion focal tyrosine kinase (RAFTK)1 – Inhibition of prosurvival NF-κB activity 2 Anti-myeloma activity of PIs and corticosteroids 1– 6 PIs and corticosteroids both inhibit NF-κB activity • Corticosteroids also enhance inhibitory effects of PIs on myeloma cell growth and mitigate infusion -related reactions 3, 4 • PIs may overcome corticosteroid treatment resistance conferred by prosurvival interleukin-6 (IL-6)2, 3: – Corticosteroid-induced apoptosis is inhibited by IL-6 and also results in protective upregulation of IL-6 receptor (IL-6 R) on myeloma cells 2, 5 – PI treatment inhibits IL-6 signaling through inhibition of NF-κB 3 Combination of PIs and corticosteroids inhibits myeloma cell growth 3, 4 PIs and corticosteroids both induce apoptosis through caspase 9 IL 6 gene transcription NF-κB cannot induce IL 6 gene transcription GR, glucocorticoid receptor; IL-6, interleukin 6; NF- κB, nuclear factor κB; PI, proteasome inhibitor; RAFTK, related adhesion focal tyrosine kinase. 10 Image adapted from Hideshima T, Anderson K. Nat Rev Cancer. 2002; 2: 927 -937. 1. Hideshima T, Anderson K. Nat Rev Cancer. 2002; 2: 927 -937. 2. Chauhan D, et al. Oncogene. 2002; 21: 1346 -1358. 3. Hideshima T, et al. Cancer Res. 2001; 61: 3071 -3076. 4. Kuhn DJ, et al. Blood. 2007; 110: 3281 -3290. 5. Chauhan D, et al. J Biol Chem. 2000; 275: 27845 -27850. 6. Greenstein S, et al. Clin Cancer Res. 2002; 8: 1681 -1694.
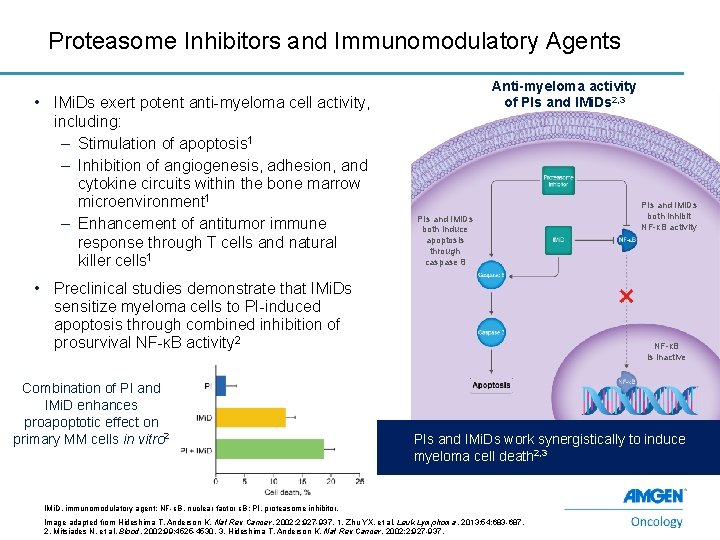
Proteasome Inhibitors and Immunomodulatory Agents • IMi. Ds exert potent anti-myeloma cell activity, including: – Stimulation of apoptosis 1 – Inhibition of angiogenesis, adhesion, and cytokine circuits within the bone marrow microenvironment 1 – Enhancement of antitumor immune response through T cells and natural killer cells 1 Anti-myeloma activity of PIs and IMi. Ds 2, 3 PIs and IMi. Ds both induce apoptosis through caspase 8 • Preclinical studies demonstrate that IMi. Ds sensitize myeloma cells to PI-induced apoptosis through combined inhibition of prosurvival NF-κB activity 2 Combination of PI and IMi. D enhances proapoptotic effect on primary MM cells in vitro 2 NF-κB is inactive PIs and IMi. Ds work synergistically to induce myeloma cell death 2, 3 IMi. D, immunomodulatory agent; NF-κB, nuclear factor κB; PI, proteasome inhibitor. 11 PIs and IMi. Ds both inhibit NF-κB activity Image adapted from Hideshima T, Anderson K. Nat Rev Cancer. 2002; 2: 927 -937. 1. Zhu YX, et al. Leuk Lymphoma. 2013; 54: 683 -687. 2. Mitsiades N, et al. Blood. 2002; 99: 4525 -4530. 3. Hideshima T, Anderson K. Nat Rev Cancer. 2002; 2: 927 -937.
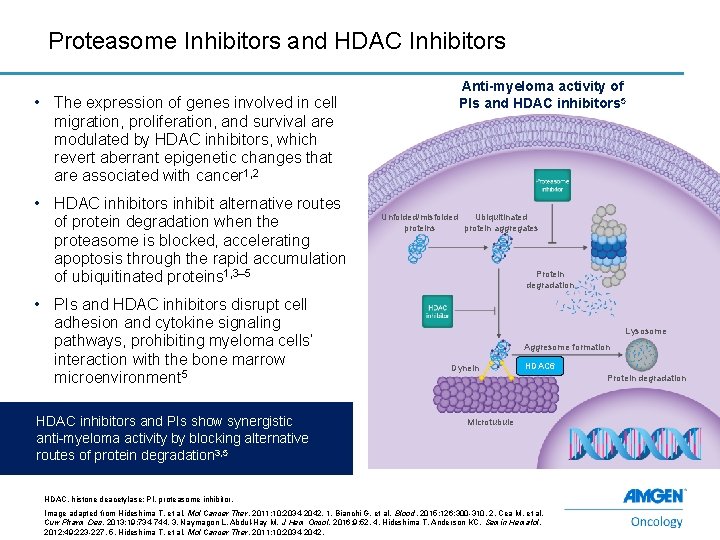
Proteasome Inhibitors and HDAC Inhibitors • The expression of genes involved in cell migration, proliferation, and survival are modulated by HDAC inhibitors, which revert aberrant epigenetic changes that are associated with cancer 1, 2 • HDAC inhibitors inhibit alternative routes of protein degradation when the proteasome is blocked, accelerating apoptosis through the rapid accumulation of ubiquitinated proteins 1, 3– 5 • PIs and HDAC inhibitors disrupt cell adhesion and cytokine signaling pathways, prohibiting myeloma cells’ interaction with the bone marrow microenvironment 5 HDAC inhibitors and PIs show synergistic anti-myeloma activity by blocking alternative routes of protein degradation 3, 5 Anti-myeloma activity of PIs and HDAC inhibitors 5 Unfolded/misfolded Ubiquitinated proteins protein aggregates Protein degradation Lysosome Aggresome formation Dynein HDAC 6 Protein degradation Microtubule HDAC, histone deacetylase; PI, proteasome inhibitor. 12 Image adapted from Hideshima T, et al. Mol Cancer Ther. 2011; 10: 2034 -2042. 1. Bianchi G, et al. Blood. 2015; 126: 300 -310. 2. Cea M, et al. Curr Pharm Des. 2013; 19: 734 -744. 3. Naymagon L, Abdul-Hay M. J Hem Oncol. 2016; 9: 52. 4. Hideshima T, Anderson KC. Semin Hematol. 2012; 49: 223 -227. 5. Hideshima T, et al. Mol Cancer Ther. 2011; 10: 2034 -2042.
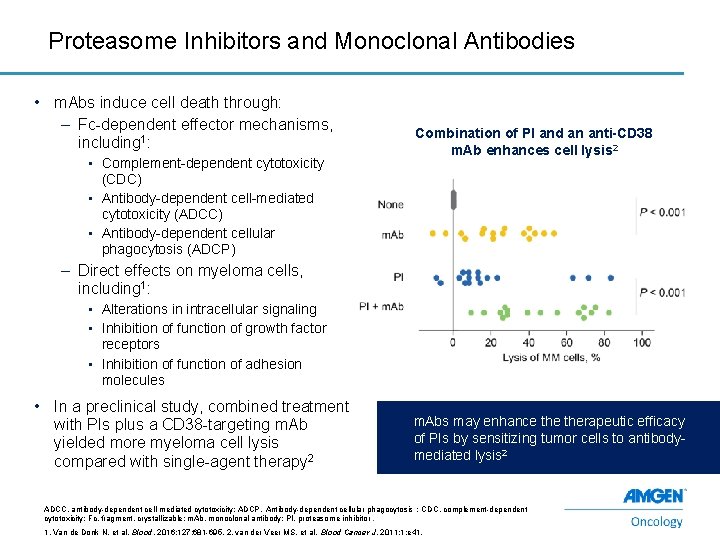
Proteasome Inhibitors and Monoclonal Antibodies • m. Abs induce cell death through: – Fc-dependent effector mechanisms, including 1: • Complement-dependent cytotoxicity (CDC) • Antibody-dependent cell-mediated cytotoxicity (ADCC) • Antibody-dependent cellular phagocytosis (ADCP) Combination of PI and an anti-CD 38 m. Ab enhances cell lysis 2 – Direct effects on myeloma cells, including 1: • Alterations in intracellular signaling • Inhibition of function of growth factor receptors • Inhibition of function of adhesion molecules • In a preclinical study, combined treatment with PIs plus a CD 38 -targeting m. Ab yielded more myeloma cell lysis compared with single-agent therapy 2 m. Abs may enhance therapeutic efficacy of PIs by sensitizing tumor cells to antibodymediated lysis 2 ADCC, antibody-dependent cell-mediated cytotoxicity; ADCP, Antibody-dependent cellular phagocytosis ; CDC, complement-dependent cytotoxicity; Fc, fragment, crystallizable; m. Ab, monoclonal antibody; PI, proteasome inhibitor. 13 1. Van de Donk N, et al. Blood. 2016; 127: 681 -695. 2. van der Veer MS, et al. Blood Cancer J. 2011; 1: e 41.
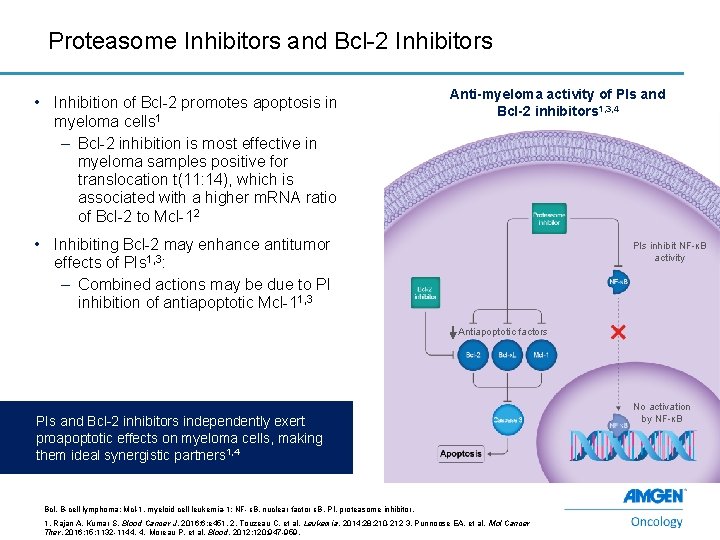
Proteasome Inhibitors and Bcl-2 Inhibitors • Inhibition of Bcl-2 promotes apoptosis in myeloma cells 1 – Bcl-2 inhibition is most effective in myeloma samples positive for translocation t(11: 14), which is associated with a higher m. RNA ratio of Bcl-2 to Mcl-12 Anti-myeloma activity of PIs and Bcl-2 inhibitors 1, 3, 4 • Inhibiting Bcl-2 may enhance antitumor effects of PIs 1, 3: – Combined actions may be due to PI inhibition of antiapoptotic Mcl-11, 3 PIs inhibit NF-κB activity Antiapoptotic factors PIs and Bcl-2 inhibitors independently exert proapoptotic effects on myeloma cells, making them ideal synergistic partners 1, 4 Bcl, B-cell lymphoma; Mcl-1, myeloid cell leukemia-1; NF-κB, nuclear factor κB, PI, proteasome inhibitor. 14 1. Rajan A, Kumar S. Blood Cancer J. 2016; 6: e 451. 2. Touzeau C, et al. Leukemia. 2014; 28: 210 -212 3. Punnoose EA, et al. Mol Cancer Ther. 2016; 15: 1132 -1144. 4. Moreau P, et al. Blood. 2012; 120: 947 -959. No activation by NF-κB
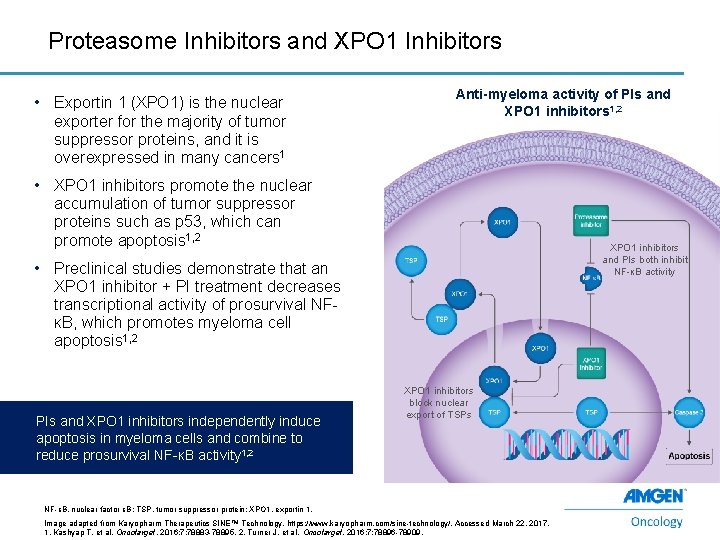
Proteasome Inhibitors and XPO 1 Inhibitors • Exportin 1 (XPO 1) is the nuclear exporter for the majority of tumor suppressor proteins, and it is overexpressed in many cancers 1 Anti-myeloma activity of PIs and XPO 1 inhibitors 1, 2 • XPO 1 inhibitors promote the nuclear accumulation of tumor suppressor proteins such as p 53, which can promote apoptosis 1, 2 XPO 1 inhibitors and PIs both inhibit NF-κB activity • Preclinical studies demonstrate that an XPO 1 inhibitor + PI treatment decreases transcriptional activity of prosurvival NFκB, which promotes myeloma cell apoptosis 1, 2 PIs and XPO 1 inhibitors independently induce apoptosis in myeloma cells and combine to reduce prosurvival NF-κB activity 1, 2 XPO 1 inhibitors block nuclear export of TSPs NF-κB, nuclear factor κB; TSP, tumor suppressor protein; XPO 1, exportin 1. 15 Image adapted from Karyopharm Therapeutics SINE™ Technology. https: //www. karyopharm. com/sine-technology/. Accessed March 22, 2017. 1. Kashyap T, et al. Oncotarget. 2016; 7: 78883 -78895. 2. Turner J, et al. Oncotarget. 2016; 7: 78896 -78909.
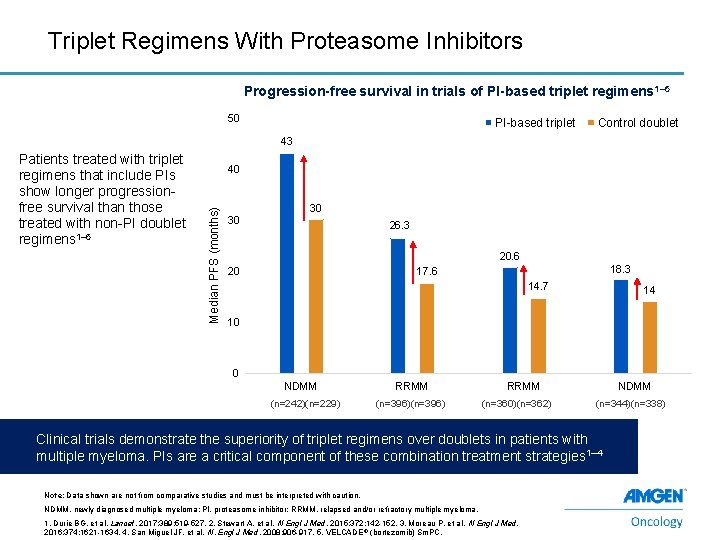
Triplet Regimens With Proteasome Inhibitors Progression-free survival in trials of PI-based triplet regimens 1– 5 50 PI-based triplet Control doublet 43 40 Median PFS (months) Patients treated with triplet regimens that include PIs show longer progressionfree survival than those treated with non-PI doublet regimens 1– 5 30 30 26. 3 20. 6 20 18. 3 17. 6 14. 7 14 10 0 NDMM (n=242)(n=229) RRMM (n=396) RRMM (n=360)(n=362) NDMM (n=344)(n=338) Clinical trials demonstrate the superiority of triplet regimens over doublets in patients with multiple myeloma. PIs are a critical component of these combination treatment strategies 1– 4 Note: Data shown are not from comparative studies and must be interpreted with caution. NDMM, newly diagnosed multiple myeloma; PI, proteasome inhibitor; RRMM, relapsed and/or refractory multiple myeloma. 16 1. Durie BG, et al. Lancet. 2017; 389: 519 -527. 2. Stewart A, et al. N Engl J Med. 2015; 372: 142 -152. 3. Moreau P, et al. N Engl J Med. 2016; 374: 1621 -1634. 4. San Miguel JF, et al. N. Engl J Med. 2008; 906 -917. 5. VELCADE ® (bortezomib) Sm. PC.
Summary The proteasome plays a critical role in maintaining cellular homeostasis through the degradation of regulatory and misfolded proteins 1 Myeloma cells produce excessive amounts of immunoglobulins and other proteins, making them more dependent on proteasome activity than other cell types and thus highly sensitive to proteasome disruption 1– 3 Proteasome inhibitors have a complex mechanism of action that causes an accumulation of misfolded proteins within the myeloma cell, triggering ER stress and apoptosis 1 The genetic heterogeneity of myeloma cells creates the need for simultaneously targeting multiple mechanisms of disease, including proteasome dependency 4, 5 Different proteasome inhibitors are distinct regarding their specificities and binding affinities for the various catalytic sites within the proteasome core 1 Combination therapies that include proteasome inhibitors are required to target the complementary pathways underlying multiple myeloma and can yield improved clinical outcomes 1, 6 ER, endoplasmic reticulum. 17 1. Moreau P, et al. Blood. 2012; 120: 947 -959. 2. Boise L, et al. Blood. 2014; 124: 1873 -1879. 3. Bianchi G, et al. Blood. 2009; 113: 3040 -3049. 4. Bianchi G, et al. Blood. 2015; 126: 300 -310. 5. Barlogie B et al. Blood. 2014; 124: 3034 -3051. 6. Lonial S, et al. J Clin Oncol. 2012; 30: 24342436.
- Slides: 17