The Role of Nurses in Early Phase Clinical

















- Slides: 28

The Role of Nurses in Early Phase Clinical Research Association of Clinical Pharmacology Units 20 th Annual Meeting Clare Hastings, Ph. D, RN, FAAN Chief Nurse Officer NIH Clinical Center October 20, 2010 Cite as: Hastings, C. The Role of Nurses in Early Phase Clinical Research, Concurrent Session, Association of Clinical Pharmacology Units 20 th Annual Meeting, Cincinnati, OH, October, 2010


NIH Clinical Center: America’s Research Hospital • Supports intramural clinical research conducted by the Institutes and Centers of the NIH • Creates and disseminates standards and innovations for conducting clinical research • Creates and demonstrates models for clinical research training and career development for all disciplines

Major Emphasis • First in human with new therapeutics • Study of patients with rare diseases

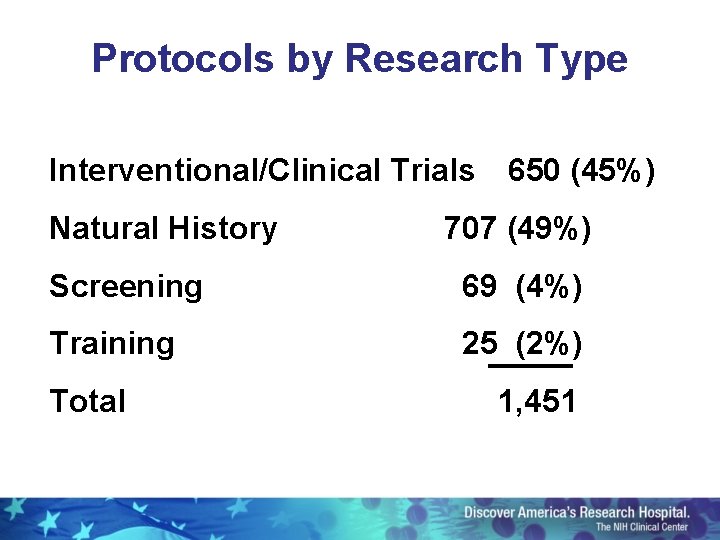
Protocols by Research Type Interventional/Clinical Trials Natural History 650 (45%) 707 (49%) Screening 69 (4%) Training 25 (2%) Total 1, 451

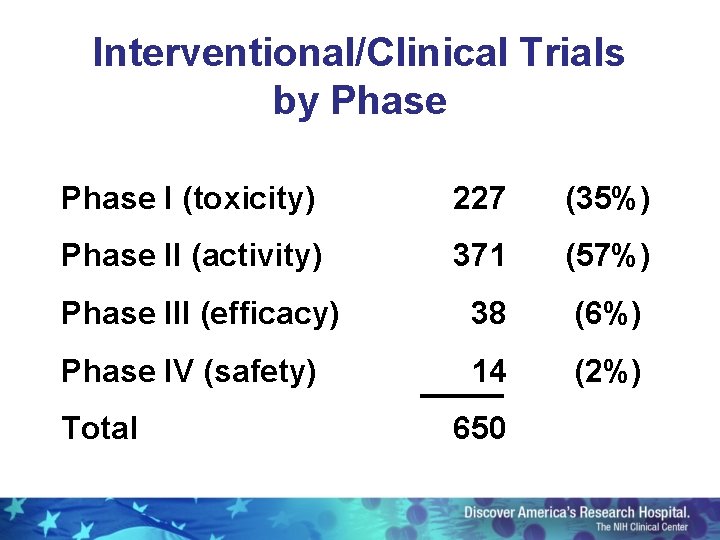
Interventional/Clinical Trials by Phase I (toxicity) 227 (35%) Phase II (activity) 371 (57%) Phase III (efficacy) 38 (6%) Phase IV (safety) 14 (2%) Total 650

Nursing Practice at the NIH Clinical Center • Acute care hospital and ambulatory care center with 634 direct care CRNs • An additional 230+ RNs work with investigators as research nurse coordinators • Staffed at a level to support precision in patient care and data collection • Clinical care requirements are protocol-driven

A Global Agenda to Define and Support the Practice of Nurses in Clinical Research

Definition of Clinical Research Nursing Clinical research nursing is nursing practice with a specialty focus on the care of research participants. 1. 2. 3. 4. 5. Providing and coordinating clinical care Assuring participant safety Ongoing maintenance of informed consent Integrity of protocol implementation Accuracy of data collection and recording Care received by research participants is driven by study requirements and the collection of research data as well as clinical indications.

Specialty Definition Steps • • Define practice domain Establish practice standards Determine core competencies Develop tools to assist clinicians, managers and educators (core curriculum, staffing standards, core courses, etc. ) • Develop certification process

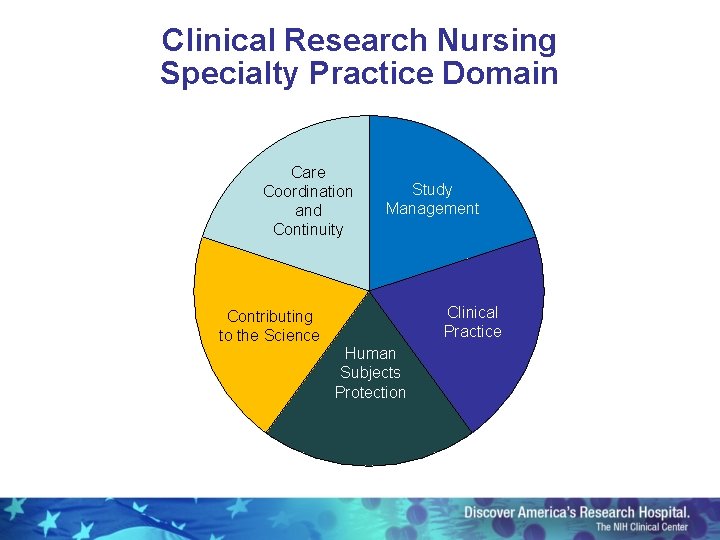
Clinical Research Nursing Specialty Practice Domain Care Coordination and Continuity Study Management Clinical Practice Contributing to the Science Human Subjects Protection

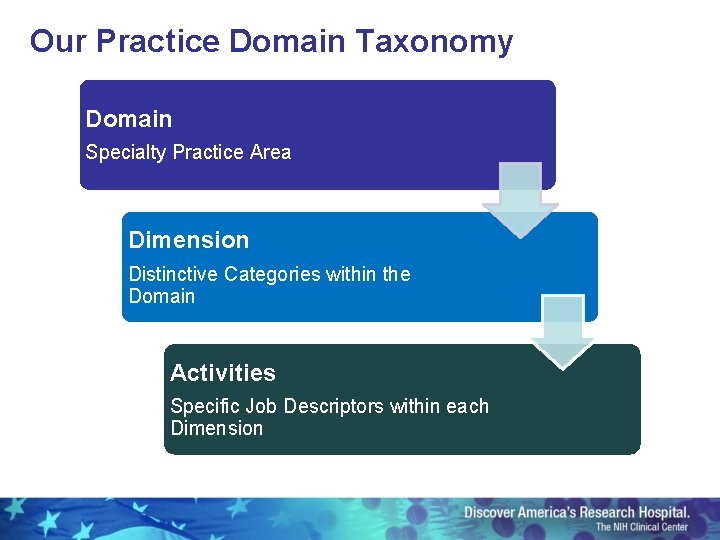
Our Practice Domain Taxonomy Domain Specialty Practice Area Dimension Distinctive Categories within the Domain Activities Specific Job Descriptors within each Dimension

Clinical Practice Provision of direct nursing care and support, using the nursing process, to participants in clinical research, their families and significant others. Care requirements are determined by the scope of study participation, the clinical condition of the patient and the requirements and clinical effects of research procedures.

Study Management of clinical and research support activities in order to assure patient safety, address clinical needs and assure protocol integrity and accurate data collection.

Care Coordination and Continuity Coordination of research and clinical activities to meet clinical needs, complete study requirements and manage linkage with referring and primary care providers.

Human Subject Protection Facilitation of informed participation by diverse participants in clinical research.

Contributing to the Science Contribution as a research team member to the development of new ideas for study and explorations of innovations arising from clinical research finding to practice.

Validating the Domain of Practice: A National Delphi Survey (2008) • National sample of nurses actively involved with clinical research • Used an electronic survey format for distribution • 3 rounds completed by 22 participants

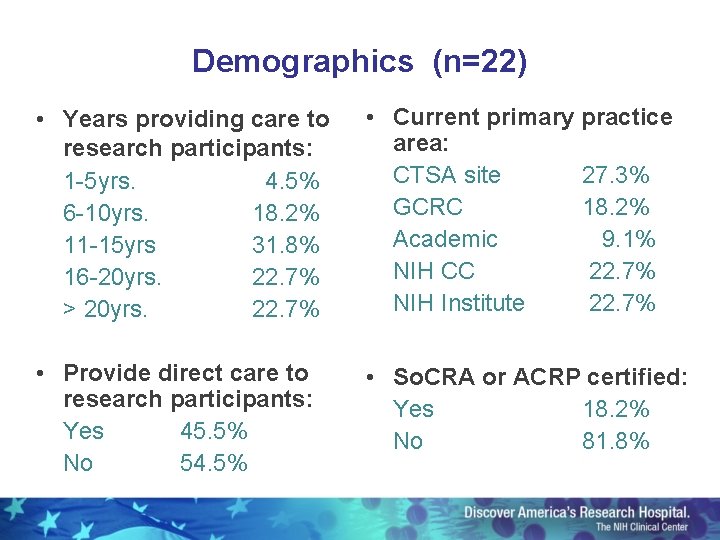
Demographics (n=22) • Years providing care to research participants: 1 -5 yrs. 4. 5% 6 -10 yrs. 18. 2% 11 -15 yrs 31. 8% 16 -20 yrs. 22. 7% > 20 yrs. 22. 7% • Current primary practice area: CTSA site 27. 3% GCRC 18. 2% Academic 9. 1% NIH CC 22. 7% NIH Institute 22. 7% • Provide direct care to research participants: Yes 45. 5% No 54. 5% • So. CRA or ACRP certified: Yes 18. 2% No 81. 8%

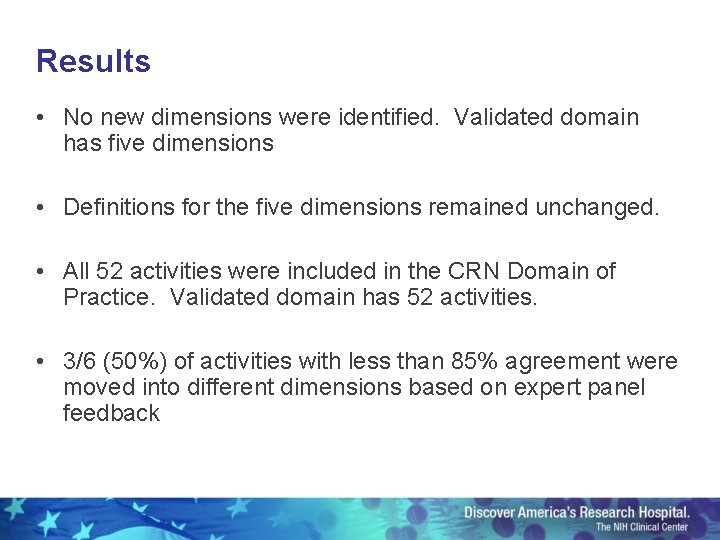
Results • No new dimensions were identified. Validated domain has five dimensions • Definitions for the five dimensions remained unchanged. • All 52 activities were included in the CRN Domain of Practice. Validated domain has 52 activities. • 3/6 (50%) of activities with less than 85% agreement were moved into different dimensions based on expert panel feedback

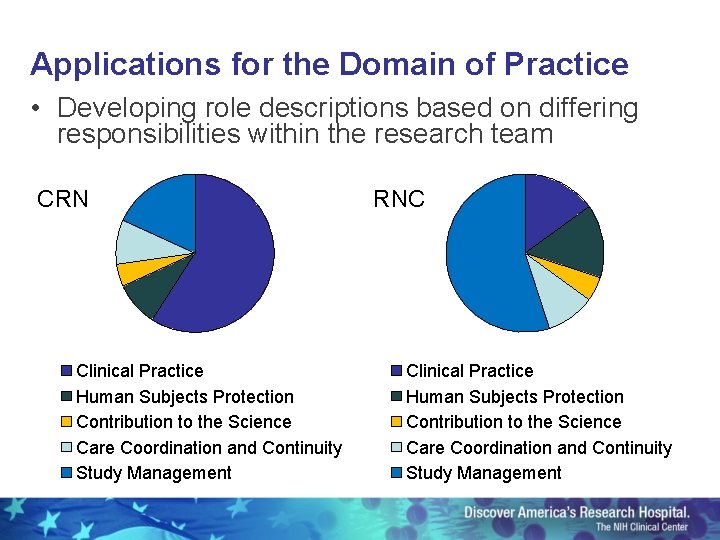
Applications for the Domain of Practice • Developing role descriptions based on differing responsibilities within the research team CRN Clinical Practice Human Subjects Protection Contribution to the Science Care Coordination and Continuity Study Management RNC Clinical Practice Human Subjects Protection Contribution to the Science Care Coordination and Continuity Study Management

“Phase I Nursing Care” Nursing implication of “first-in-humans” studies • Developing “mechanism-based” strategies to promote patient safety • Developing techniques for managing and evaluating treatment delivery systems that have never been attempted in humans before • Learning practical clinical implications of new modalities • Understanding quality of life implications for new treatments for advanced or incurable disease • Developing strategies for informed consent and patient education

CLINICAL PRACTICE DIMENSION CP 1 Provide direct nursing care to research participants (Example: interact with research participants to provide nursing care, administration of research interventions, specimen collection, etc. ) CP 2 Provide teaching to research participants and family regarding study participation, participant’s current clinical condition, and/or disease process CP 3 Monitor the research participant and report potential adverse events to a member of the research team CP 4 Record research data (Example: document vital signs, administration of a research compound, participant responses, etc. ) in approved source document (Example: the medical record, data collection sheet, etc. )

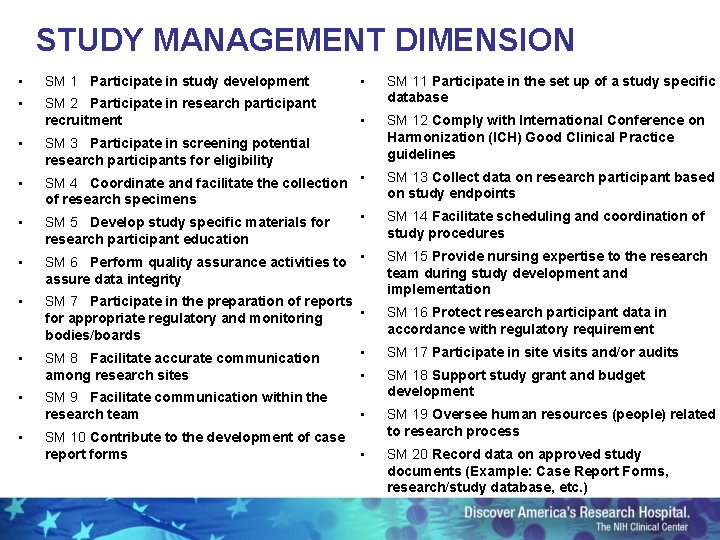
STUDY MANAGEMENT DIMENSION • SM 1 Participate in study development • • SM 2 Participate in research participant recruitment SM 11 Participate in the set up of a study specific database • SM 12 Comply with International Conference on Harmonization (ICH) Good Clinical Practice guidelines • SM 3 Participate in screening potential research participants for eligibility • SM 4 Coordinate and facilitate the collection • of research specimens • SM 5 Develop study specific materials for • research participant education • SM 6 Perform quality assurance activities to • assure data integrity • SM 7 Participate in the preparation of reports • for appropriate regulatory and monitoring bodies/boards • SM 8 Facilitate accurate communication • • • SM 13 Collect data on research participant based on study endpoints SM 14 Facilitate scheduling and coordination of study procedures SM 15 Provide nursing expertise to the research team during study development and implementation SM 16 Protect research participant data in accordance with regulatory requirement SM 17 Participate in site visits and/or audits among research sites • SM 9 Facilitate communication within the research team SM 18 Support study grant and budget development • SM 10 Contribute to the development of case report forms SM 19 Oversee human resources (people) related to research process • SM 20 Record data on approved study documents (Example: Case Report Forms, research/study database, etc. )

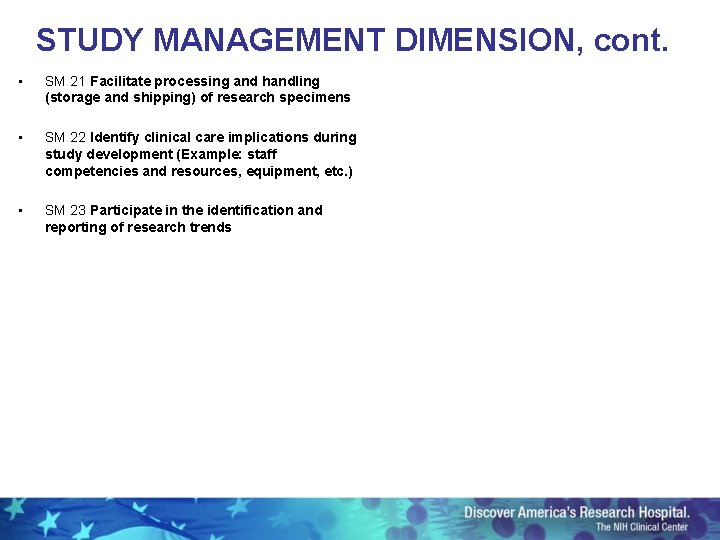
STUDY MANAGEMENT DIMENSION, cont. • SM 21 Facilitate processing and handling (storage and shipping) of research specimens • SM 22 Identify clinical care implications during study development (Example: staff competencies and resources, equipment, etc. ) • SM 23 Participate in the identification and reporting of research trends

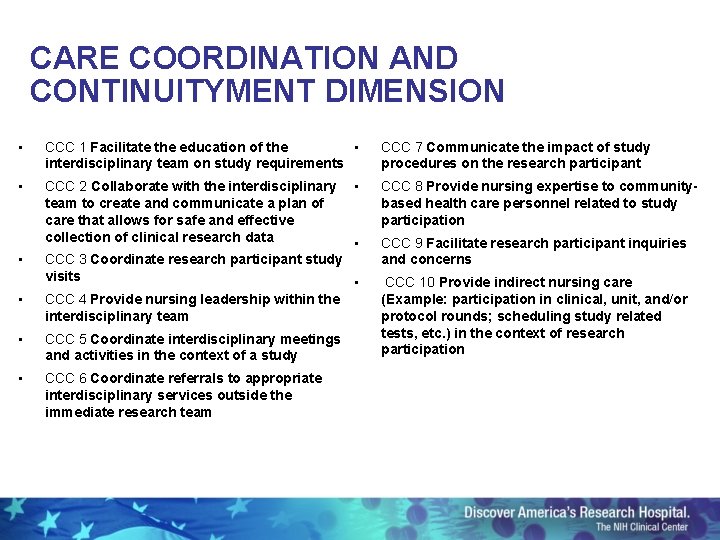
CARE COORDINATION AND CONTINUITYMENT DIMENSION • CCC 1 Facilitate the education of the • interdisciplinary team on study requirements • CCC 2 Collaborate with the interdisciplinary team to create and communicate a plan of care that allows for safe and effective collection of clinical research data • • • CCC 8 Provide nursing expertise to communitybased health care personnel related to study participation • CCC 9 Facilitate research participant inquiries and concerns CCC 3 Coordinate research participant study visits • CCC 4 Provide nursing leadership within the interdisciplinary team • CCC 5 Coordinate interdisciplinary meetings and activities in the context of a study • CCC 6 Coordinate referrals to appropriate interdisciplinary services outside the immediate research team CCC 7 Communicate the impact of study procedures on the research participant CCC 10 Provide indirect nursing care (Example: participation in clinical, unit, and/or protocol rounds; scheduling study related tests, etc. ) in the context of research participation

HUMAN SUBJECTS PROTECTION DIMENSION • HSP 1 Facilitate the initial and ongoing informed consent/assent process • HSP 2 Support research participant in defining his/her reasons and goals for participating in a study • HSP 3 Collaborate with the interdisciplinary team to address ethical conflicts • HSP 4 Coordinate research activities to minimize subject risk • HSP 5 Serve as IRB member • HSP 6 Manage potential ethical and financial conflicts of interest for self

CONTRIBUTING TO THE SCIENCE DIMENSION • CS 1 Disseminate clinical expertise and best practices related to clinical research through presentations, publications and/or interactions with nursing colleagues • CS 2 Serve as an expert in a specialty area (Example: grant reviewer, editorial board, presenter, etc. ) • CS 3 • CS 4 Generate practice questions as a result of a new study procedure or intervention • CS 5 Collaborate with the interdisciplinary team to develop innovations in care delivery that have the potential to improve patient outcomes and accuracy of data collection • CS 6 Identify questions appropriate for clinical nursing research as a result of study team participation • CS 7 team Mentor junior staff and students participating as members of the research • CS 8 ideas Perform secondary data analysis to contribute to the development of new • CS 9 Serve as a resource to new investigator Participate in the query and analysis of research data