The Role of Noninvasive Hemodynamic Monitoring in Goal





















- Slides: 52

The Role of Non-invasive Hemodynamic Monitoring in Goal Directed Fluid Management. Kimberly Sacco Duke University

Objectives • Provides information about the evolution of hemodynamic monitoring from invasive to the more recent noninvasive devices. • Review current evidence on the efficacy of Goal Directed Therapy • Review how these devices can be used in the operating rooms through well defined algorithms of goal directed therapy. • Open a discussion on SRNA experiences with these monitors and the overall reception among anesthesia providers.

Introduction • Approximately 240 million anesthesia procedures are performed annually (world wide) • Of these, 10% of high risk cases contribute to 80% of the overall mortality related to surgery. • Moderate risk surgery represents 40% of the annual total. • It is estimated that 30% of moderate risk surgeries result with a minor postoperative complication.

Introduction • Many of the minor postoperative complications are related to tissue hypoperfusion, i. e. delayed wound healing, PONV, Gut hypoperfusion leading to delayed enteral feeding. • Minor complications result in more postoperative treatment, longer length of stay and increased overall cost.

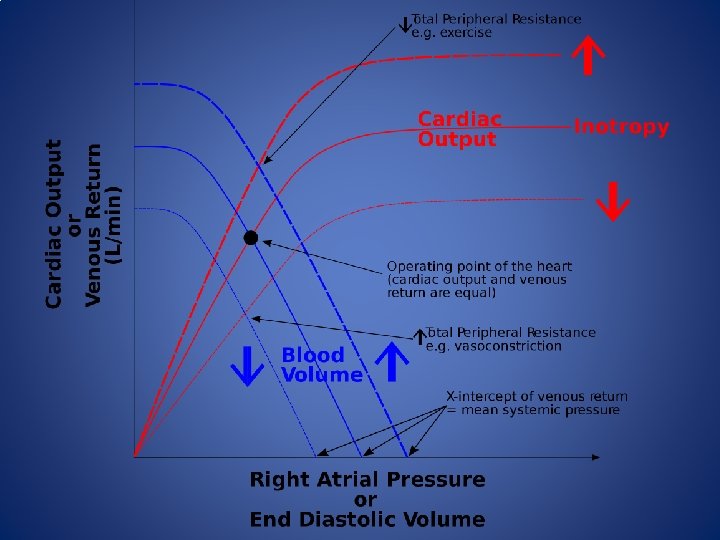
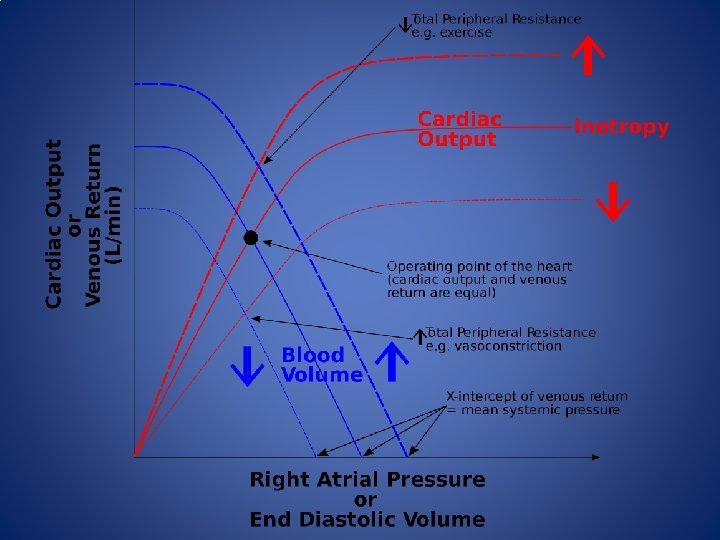
Goal Directed Therapy • An important goal of anesthesia providers is to maintain tissue perfusion through the optimization of intravascular volume and stroke volume • Normal tissue perfusion in the words of Guyton: “An adequate perfusion pressure in order to force blood into the capillaries of all organs and an adequate cardiac output to deliver oxygen and substrates and to remove carbon dioxide and other metabolic products”

Goal Directed Therapy • Fluid therapy in the OR is traditionally guided by HR, BP, urine output, peripheral SPO 2 and CVP. Fluid administration is also guided by the 4 -2 -1 method (1950 s). • Goal directed therapy (GDT) uses the manipulation of physiologic targets to guide intravenous fluid administration and inotropic therapy to improve CO and oxygen delivery and to maintain euvolemia. • Several devices, invasive and noninvasive, exist that can provide cardiac output (CO) monitoring, Stroke Volume (SV), Pule Pressure Variation (PPV) and (Stroke Volume Variation).


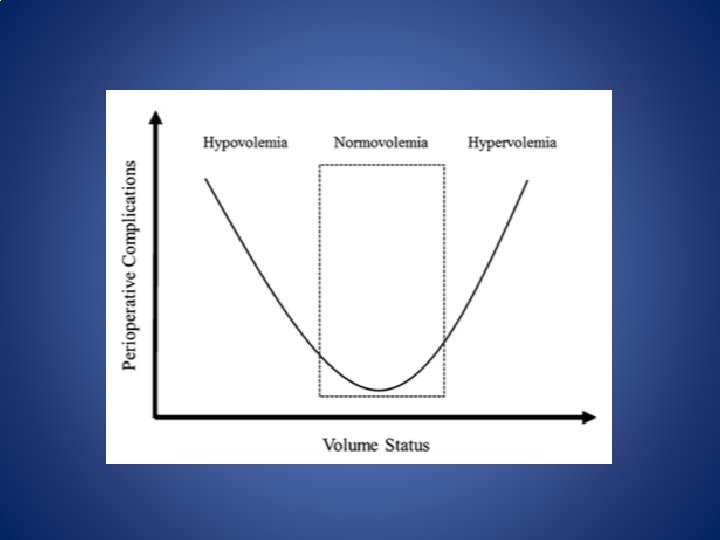
Goal Directed Therapy • Hypervolemia – Increases venous pressure leading to peripheral edema – Increased O 2 demand – Decreased tissue oxygenation – Can cause hemodilution • Hypovolemia – Reduces effective blood volume and diverts blood flow from non-vital organs – Activates SNS and Reninangiotensin system – Increases inflammatory response


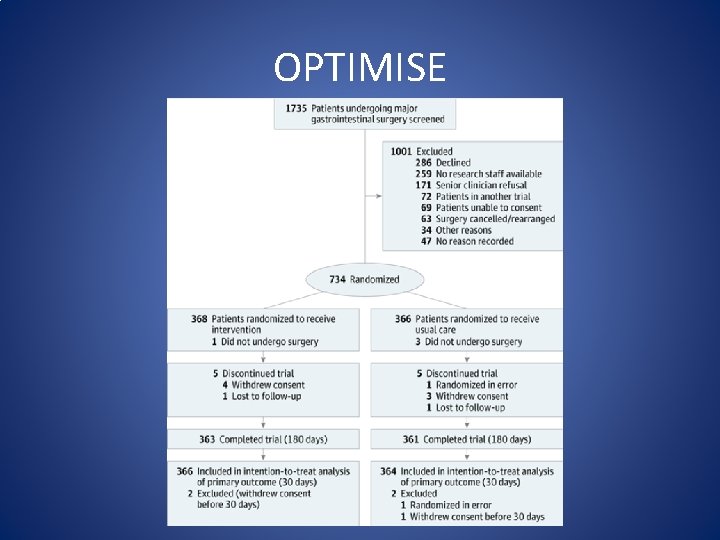
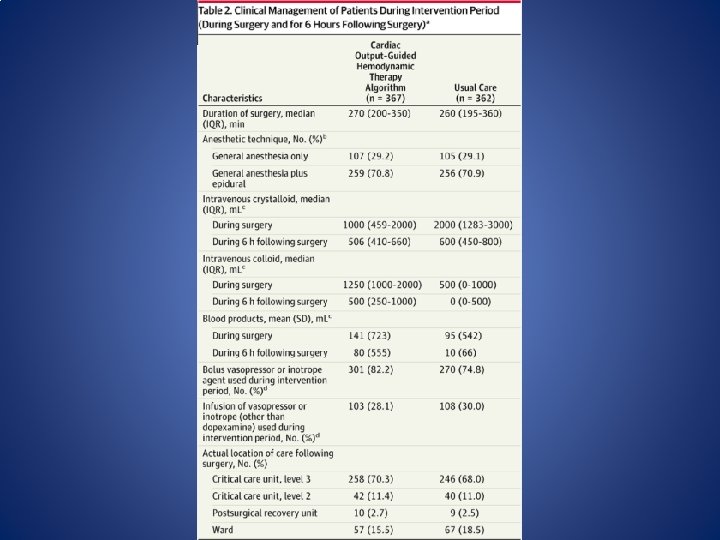
OPTIMISE (Optimisation of Cardiovascular Management to Improve Surgical Outcome) • Pearse et al. conducted a randomized clinical trial in 734 high-risk patients undergoing emergency major gastrointestinal surgery. Patients in the intervention group received a 250 ml colloid fluid bolus over 5 minutes as long as the stroke volume was responsive (changing more than 10%). CO SV and SVV were measured by the Li. DCO rapid system. Pearse RM, Harrison DA, Mac. Donald N, Gillies MA, Blunt M, Ackland G et al. Effect of a perioperative, cardiac output- guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014; 311: 2181– 90.

OPTIMISE


OPTIMISE (Optimisation of Cardiovascular Management to Improve Surgical Outcome) • The study revealed that GDT using CO guided hemodynamic management was related to a reduction in postoperative complications (intervention 36. 6 %, control 43. 4%; relative risk = 0. 84, 95 % confidence interval 0. 71 to 1. 01). However, findings were not statistically significant. Pearse RM, Harrison DA, Mac. Donald N, Gillies MA, Blunt M, Ackland G et al. Effect of a perioperative, cardiac output- guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014; 311: 2181– 90.

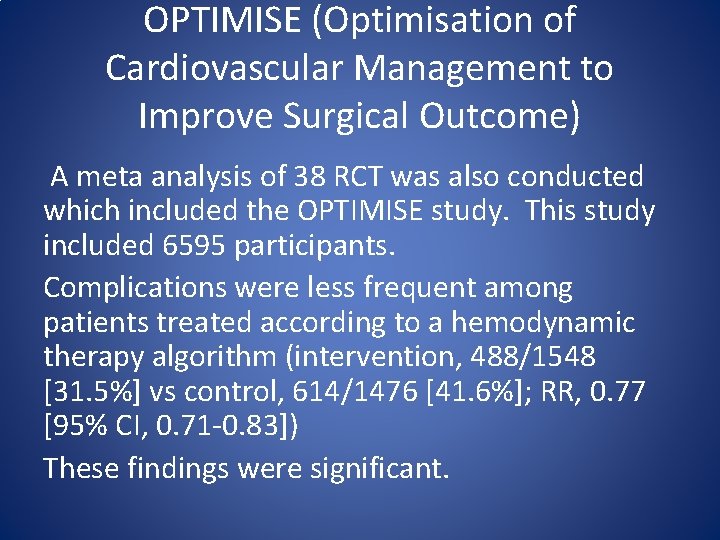
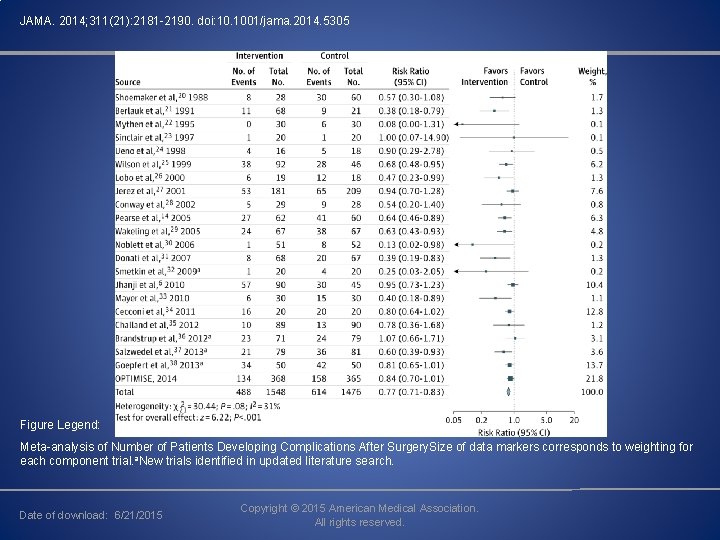
OPTIMISE (Optimisation of Cardiovascular Management to Improve Surgical Outcome) A meta analysis of 38 RCT was also conducted which included the OPTIMISE study. This study included 6595 participants. Complications were less frequent among patients treated according to a hemodynamic therapy algorithm (intervention, 488/1548 [31. 5%] vs control, 614/1476 [41. 6%]; RR, 0. 77 [95% CI, 0. 71 -0. 83]) These findings were significant.

JAMA. 2014; 311(21): 2181 -2190. doi: 10. 1001/jama. 2014. 5305 Figure Legend: Meta-analysis of Number of Patients Developing Complications After Surgery. Size of data markers corresponds to weighting for each component trial. a. New trials identified in updated literature search. Date of download: 6/21/2015 Copyright © 2015 American Medical Association. All rights reserved.

Hemodynamic Monitoring

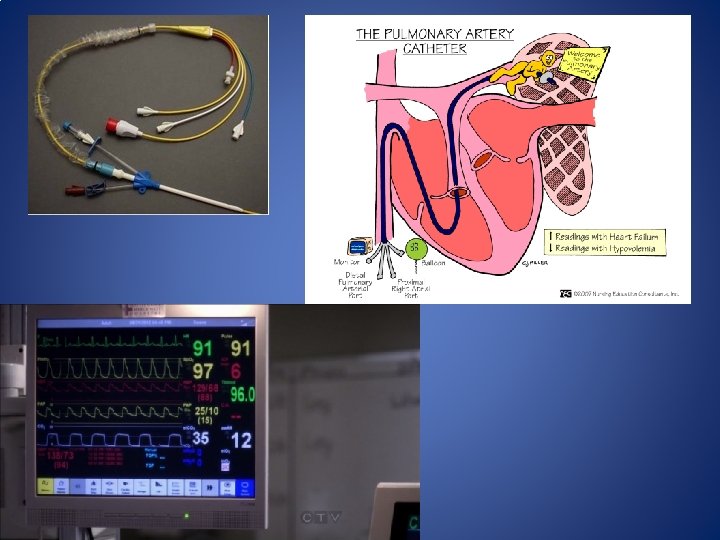
Invasive Hemodynamic monitoring • Pulmonary Artery Catheter – Measures central venous, right-sided intracardiac, pulmonary arterial and pulmonary artery wedge pressures. – Estimates CO, SVR and PVR – Allows for Sv. O 2 measurement – Considered the reference against which non-invasive devices are compared. – 12 -15% variance from CO dilution method. – Invasive monitor • Risk of infection, arrhythmias, VAE, Pneumothorax and pulmonary artery rupture.


Invasive Hemodynamic monitoring • Pulse Contour Analysis – Uses software to run algorithms based on the relationship between SV, peripheral vascular resistance, arterial pressure and arterial compliance. – Provides an estimated measure of CO, SVV, PPV – Requires Arterial Line – Requires the tidal volume of 6 ml/kg, NSR, and experiencing normal intra-abdominal pressure.

Invasive Hemodynamic monitoring • Pulse Contour Analysis – Some devices require dilution CO calibration (requires central access) • Pi. CCO: Pulsion Medical Systems, Munish, Germany • Volume view: Edwards Lifesciences, Irvine, CA, USA • Li. DCO plus: Li. DCO, London, UK – Other devices require no calibration • • Li. DCO rapid: Li. DCO, London, UK Vigileo-Flo. Trac: Edwards Lifesciences, Irvine, CA, USA PRAM: Vyetech Health, Padua, Italy Pulsioflex: Pulsion Medical Systems, Munish, Germany


Non-invasive Monitoring • Non-calibrated Pulse Contour Analysis – Nexfin: Edward lifesciences – Uses finger cuffs that enable continuous measurement of arterial pressure – Estimates CO, SVV • Estimates CO in two steps – Utilizes photoplethysmographic device that measures the diameter of the arteries in the finger and inflates to a constant pressure. – Then it estimates CO based on a pulse contour analysis


Non-Invasive Monitoring • Ultrasound – Ultrasound estimates the velocity of a moving object by analyzing the difference in frequency between the incident and reflected sound waves. – Doppler equation Where (v) the velocity of the target, the incident angle, (c) speed of sound in the medium, (f) the frequency difference, and (f 0) the frequency of the originally emitted ultrasound beam.

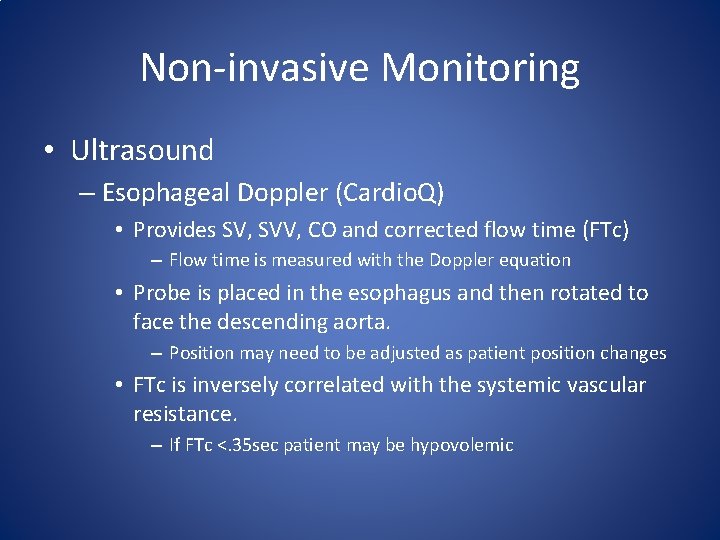
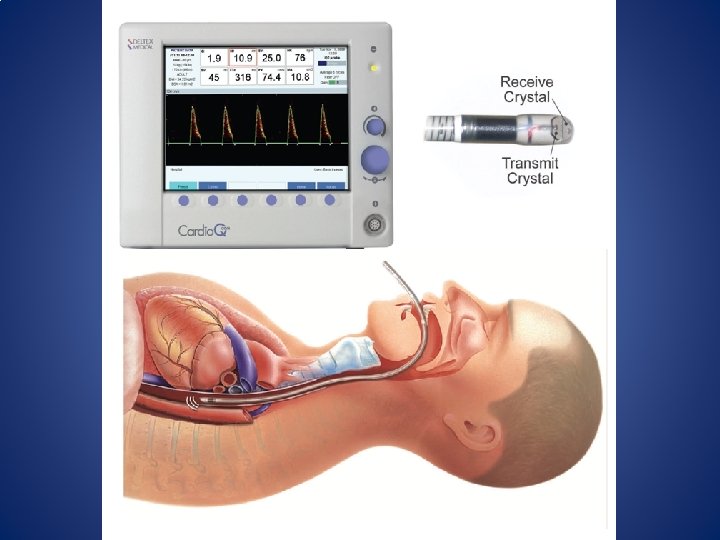
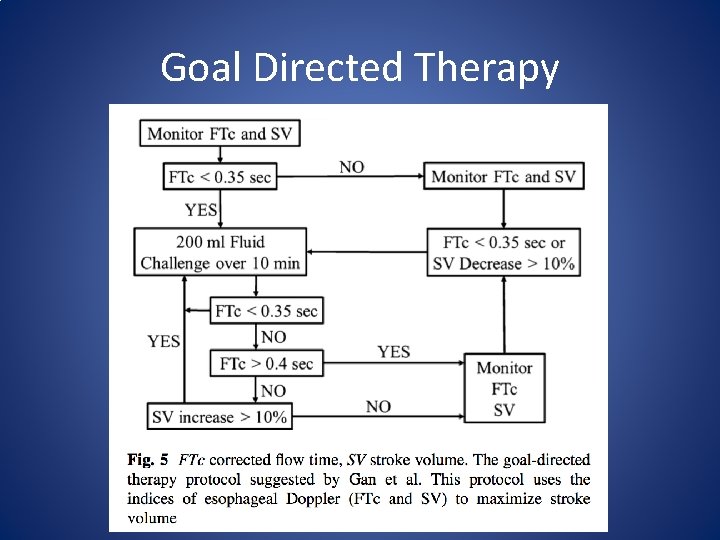
Non-invasive Monitoring • Ultrasound – Esophageal Doppler (Cardio. Q) • Provides SV, SVV, CO and corrected flow time (FTc) – Flow time is measured with the Doppler equation • Probe is placed in the esophagus and then rotated to face the descending aorta. – Position may need to be adjusted as patient position changes • FTc is inversely correlated with the systemic vascular resistance. – If FTc <. 35 sec patient may be hypovolemic

Non-invasive Monitoring • Ultrasound – Esophageal Doppler can be misleading in critically ill patients as although there may be a responsive change in fluid status in the aorta, a disproportionate amount of blood is shunted through the carotid arteries.


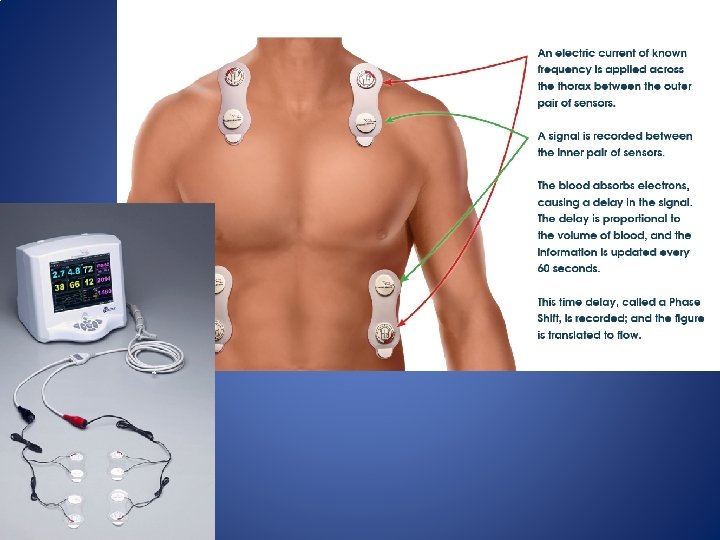
Non-invasive Monitoring • Thoracic Electrical Bioimpedance – Founded on the concept that electrical conduction within the thoracic space varies with the amount of blood within the thoracic space. – CO measurements are calculated by measuring changes in flow (I) and thoracic impedance (Z) based on its relationship to electrical potential difference (E) as defined by Ohm’s law: I=E/Z

Non-invasive Monitoring • Limitations of Bioimpedence – Varience in electric skin conductivity between the body and electrodes. (affected by temperature and humidity) – Patient movement and bovie use interfere with bioimpedence

Non-Invasive Monitoring • Bioreactance – Based on the discovery that changes in aortic blood volume induce small changes in frequency of electrical signal propagation across the thorax. – Bioreactance is nearly 100 times more accurate than bioimpedance technology. – The Cheetah (NICOM) uses an algorithm that measures phase shifts in high frequency waves transmitted across the thorax. – Cheetah is as accurate in tests that compare to PAC values as esophageal doppler


Using Hemodynamic Data

Using Hemodynamic data • Current Anesthesiology Report published an article in December 2014 titled Guiding Goal. Directed Therapy by Suehiro, Joosten, Alexander and Cannesson. – Outlines a detailed guide to goal-directed therapy and a number of algorithms to aid in the interpretation of data obtained from hemodynamic monitors.

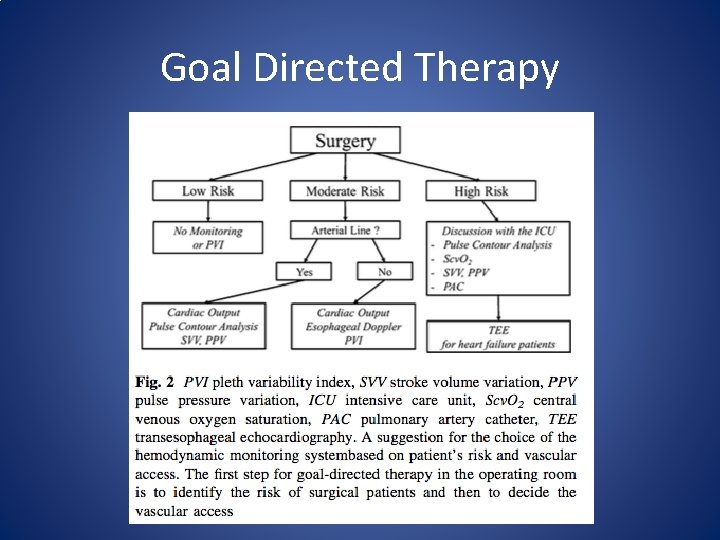
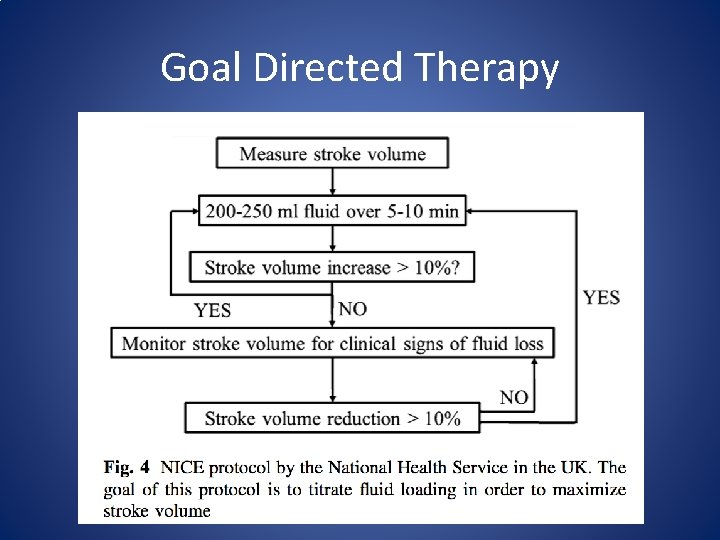
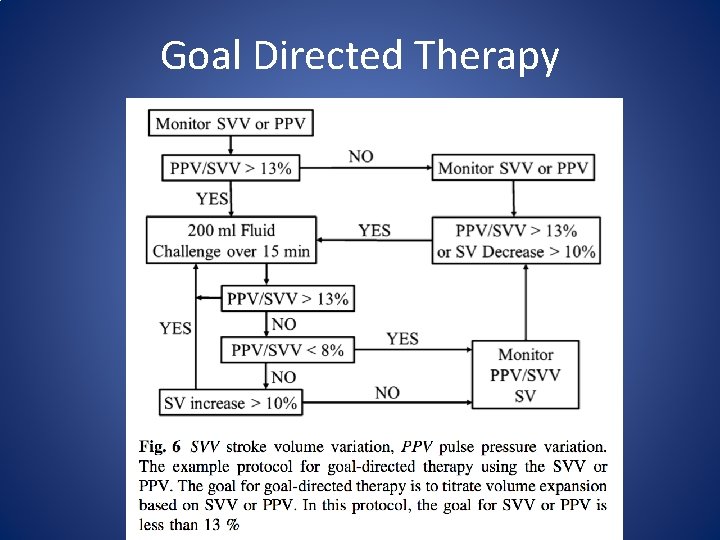
Goal Directed Therapy


Goal Directed Therapy

Goal Directed Therapy

Goal Directed Therapy

Emerging Technology

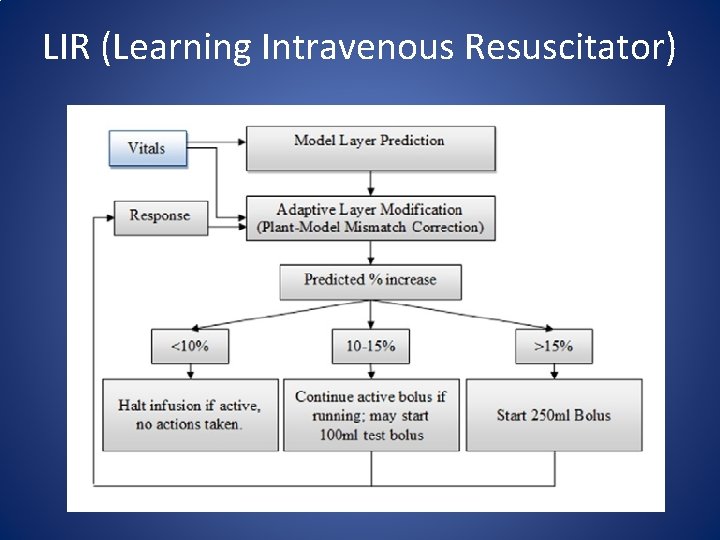
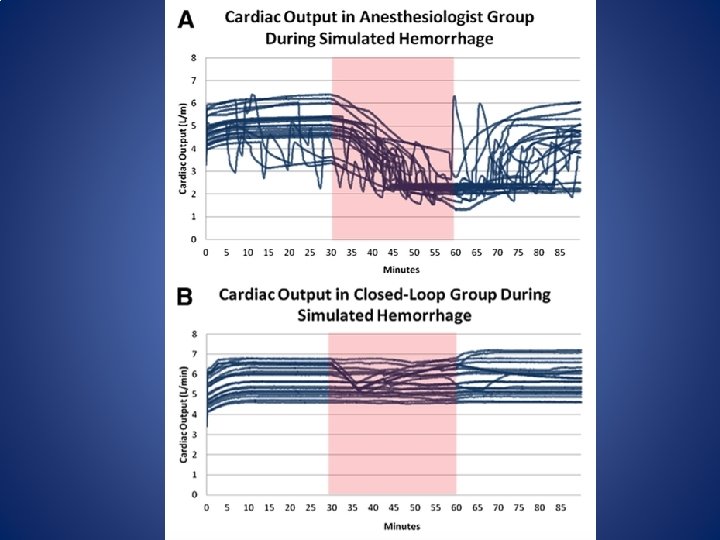
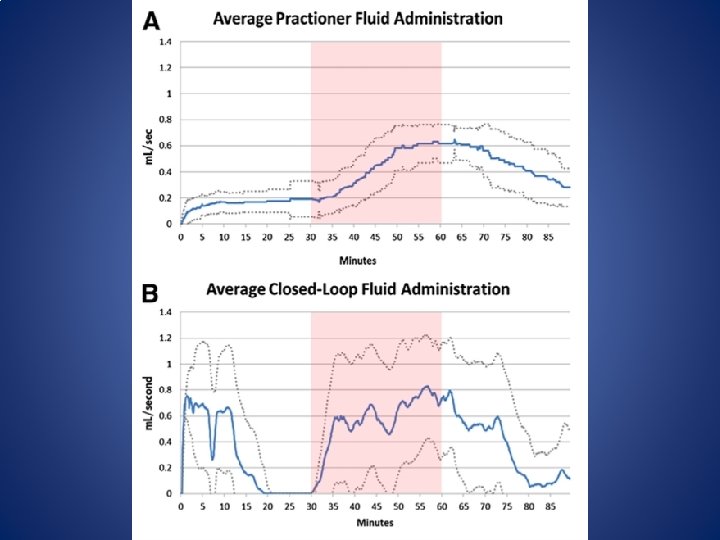
Closed Loop GDT • In 2012 Rinehart, Chung, Canales & Cannesson published a Prospective cohort study to compare the ability of LIR (Learning Intravenous Resuscitator) with anesthesiologists in optimizing CO in a simulated environment. • Rinehart, J. , Chung, E. , Canales, C. , & Cannesson, M. (October 01, 2012). Intraoperative Stroke Volume Optimization Using Stroke Volume, Arterial Pressure, and Heart Rate: Closed-Loop (Learning Intravenous Resuscitator) Versus Anesthesiologists. Journal of Cardiothoracic and Vascular Anesthesia, 26, 5, 933 -939.

LIR (Learning Intravenous Resuscitator)

Closed loop GDT – Twenty Anesthesiologists participated (10 residents, 10 attending physicians) – Simulated patients started with a fluid deficit of 15% below optimization to simulate NPO status. – Study protocol ran 90 min – At 30 min the patient began to loose 40 ml of blood per minute for 30 min (1200 ml blood loss) – Simulated patients were otherwise healthy.

Closed Loop GDT


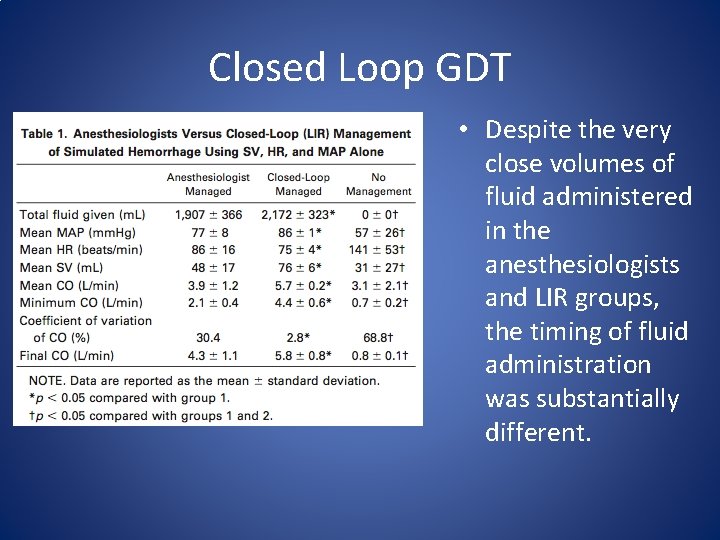
Closed Loop GDT • Despite the very close volumes of fluid administered in the anesthesiologists and LIR groups, the timing of fluid administration was substantially different.

Closed Loop GDT • Limitations: – Small sample size – Environment prone to bias. Devoid of clinical cues to help guide management decisions. – Practitioners were allowed fluid (colloid or crystalloid) as well as pressers and inotropes, whereas the LIR was only allowed fluid. – Real world testing required.

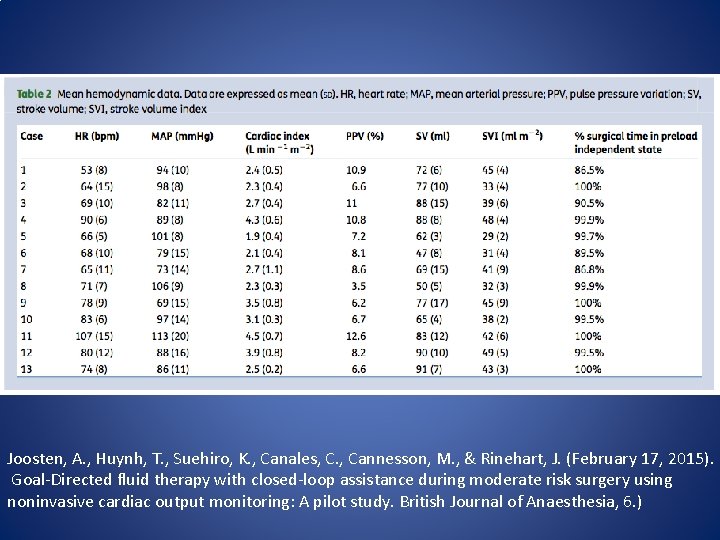
Closed loop GDT • In 2015 a prospective pilot study was published in the British Journal of Anaesthesia which evaluated the clinical feasibility of GDFT using a closed-loop fluid administration system with a non-invasive cardiac output monitoring device (Nexfin. TM, BMEYE, Amsterdam, Netherlands). • Joosten, A. , Huynh, T. , Suehiro, K. , Canales, C. , Cannesson, M. , & Rinehart, J. (February 17, 2015). Goal-Directed fluid therapy with closed-loop assistance during moderate risk surgery using noninvasive cardiac output monitoring: A pilot study. British Journal of Anaesthesia, 6. )

Closed loop GDT • Qualified patients were undergoing general anesthesia for elective moderate risk surgery • The anesthesia team selected GDFT targets and all patients received a baseline 3 ml/kg crystalloid infusion. • Colloid solutions were delivered using data from the Nexfin. TM monitor. • The goal was for the patient spent more than 85% of the surgery time in a preload independent state (defined as pulse pressure variation <13%)

Joosten, A. , Huynh, T. , Suehiro, K. , Canales, C. , Cannesson, M. , & Rinehart, J. (February 17, 2015). Goal-Directed fluid therapy with closed-loop assistance during moderate risk surgery using noninvasive cardiac output monitoring: A pilot study. British Journal of Anaesthesia, 6. )

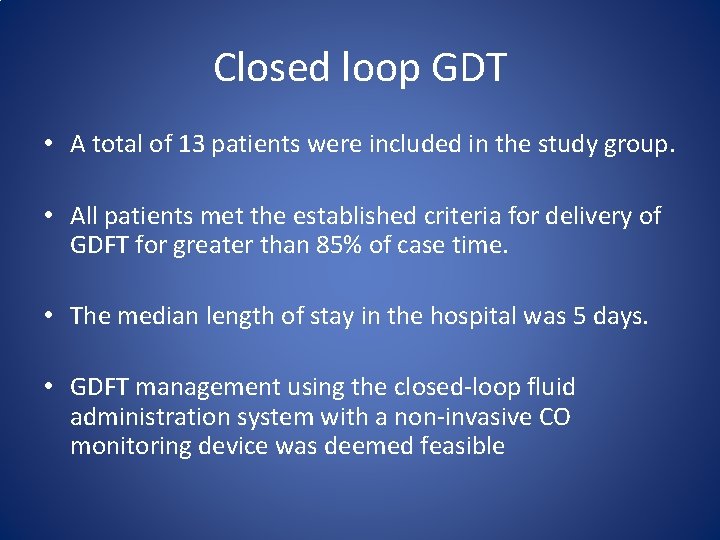
Closed loop GDT • A total of 13 patients were included in the study group. • All patients met the established criteria for delivery of GDFT for greater than 85% of case time. • The median length of stay in the hospital was 5 days. • GDFT management using the closed-loop fluid administration system with a non-invasive CO monitoring device was deemed feasible

The End Questions?

Reference: Benes, Jan, Giglio, Mariateresa, Brienza, Nicola, & Michard, Frederic. (2014). The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. (Bio. Med Central Ltd. ) Bio. Med Central Ltd. Buettner M, Schummer W, Huettemann E, Schenke S, van Hout N, Sakka SG. Influence of systolic-pressure variation-guided intraoperative fluid management on organ function and oxygen transport. Br J Anaesth. 2008; 101: 194– 9. Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM et al. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013; 17: 209. Gallagher, K. , & Vacchiano, C. (January 01, 2014). Reexamining traditional intraoperative fluid administration: evolving views in the age of goaldirected therapy. Aana Journal, 82, 3, 235 -42. Guinot, P. G. , de, B. B. , Bernard, E. , Arab, O. A. , Lorne, E. , & Dupont, H. (December 22, 2013). Respiratory stroke volume variation assessed by oesophageal Doppler monitoring predicts fluid responsiveness during laparoscopy. British Journal of Anaesthesia, 10. ) Joosten, A. , Huynh, T. , Suehiro, K. , Canales, C. , Cannesson, M. , & Rinehart, J. (February 17, 2015). Goal-Directed fluid therapy with closed-loop assistance during moderate risk surgery using noninvasive cardiac output monitoring: A pilot study. British Journal of Anaesthesia, 6. ) Kim, S. H. , Lilot, M. , Sidhu, K. S. , Rinehart, J. , Yu, Z. , Canales, C. , & Cannesson, M. (January 01, 2014). Accuracy and precision of continuous noninvasive arterial pressure monitoring compared with invasive arterial pressure: a systematic review and meta-analysis. Anesthesiology, 120, 5, 1080 -97. Navarro, L. H. C. , Bloomstone, J. A. , Auler, J. O. C. , Cannesson, M. , Rocca, G. D. , Gan, T. J. , Kinsky, M. , . . . Kramer, G. C. (December 10, 2014). Perioperative fluid therapy: a statement from the international Fluid Optimization Group. Perioperative Medicine, 4, 1. ) Pestaña, D. , Espinosa, E. , Eden, A. , Nájera, D. , Collar, L. , Aldecoa, C. , Higuera, E. , . . . Pizov, R. (January 01, 2014). Perioperative goal-directed hemodynamic optimization using noninvasive cardiac output monitoring in major abdominal surgery: a prospective, randomized, multicenter, pragmatic trial: POEMAS Study (Perioperative goal-directed therapy in Major Abdominal Surgery). Anesthesia and Analgesia, 119, 3, 579 -87. Pearse RM, Harrison DA, Mac. Donald N, Gillies MA, Blunt M, Ackland G et al. Effect of a perioperative, cardiac output- guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014; 311: 2181– 90. Rinehart, J. , Chung, E. , Canales, C. , & Cannesson, M. (October 01, 2012). Intraoperative Stroke Volume Optimization Using Stroke Volume, Arterial Pressure, and Heart Rate: Closed-Loop (Learning Intravenous Resuscitator) Versus Anesthesiologists. Journal of Cardiothoracic and Vascular Anesthesia, 26, 5, 933 -939. Rinehart J, Lee C, Canales C, Kong A, Kain Z, Cannesson M. Closed-loop fluid administration compared to anesthesiologist management for hemodynamic optimization and resuscitation during surgery: an in vivo study. Anesth Analg. 2013; 117: 1119– 29. Suehiro, K. , Joosten, A. , Alexander, B. , & Cannesson, M. (December 14, 2014). Guiding Goal-Directed Therapy. Current Anesthesiology Reports, 4, 4, 360 -375. Trinooson, C. D. , & Gold, M. E. (January 01, 2013). Impact of goal-directed perioperative fluid management in high-risk surgical procedures: a literature review. Aana Journal, 81, 5, 357 -68. Walsh, S. R. , Tang, T. , Bass, S. , & Gaunt, M. E. (January 01, 2008). Doppler-guided intra-operative fluid management during major abdominal surgery: systematic review and meta-analysis. International Journal of Clinical Practice, 62, 3, 466 -70.
 Hemodynamic monitoring system
Hemodynamic monitoring system Hemodynamic monitoring definition
Hemodynamic monitoring definition Pinsky hemodynamic monitoring
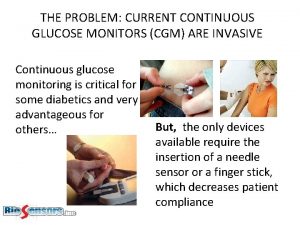
Pinsky hemodynamic monitoring Non invasive cgm
Non invasive cgm Hemodynamic disorders
Hemodynamic disorders Cerebral infarction
Cerebral infarction Hemodynamic disorders
Hemodynamic disorders Hemodynamic disorders
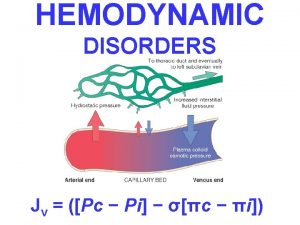
Hemodynamic disorders Normal hemodynamic values
Normal hemodynamic values Hemodynamic disorders
Hemodynamic disorders Thrombosis pathology
Thrombosis pathology Company logo
Company logo Hemodynamic disorders
Hemodynamic disorders Grasp goal role audience
Grasp goal role audience Krappmann role taking
Krappmann role taking Azure web role worker role example
Azure web role worker role example Statuses and their related roles determine the structure
Statuses and their related roles determine the structure Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Dot
Dot Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ Fecboak
Fecboak Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Ng-html
Ng-html Sơ đồ cơ thể người
Sơ đồ cơ thể người Số nguyên tố là số gì
Số nguyên tố là số gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Tư thế worm breton
Tư thế worm breton ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Cái miệng nó xinh thế
Cái miệng nó xinh thế Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Thể thơ truyền thống
Thể thơ truyền thống Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng 101012 bằng
101012 bằng