The role of MCAZ as the Regulatory Centre
































- Slides: 33

The role of MCAZ as the Regulatory Centre of excellence(RCORE) For QA/QC as designated By the NEPAD agency Protecting Your Right to Quality Medicines and Medical Devices

Presentation outline • MCAZ Lab background • Background to RCORES establishment • Criteria for Eligibility • Selection Process • MCAZ role as RCORE for QA/QC as designated by NEPAD Protecting Your Right to Quality Medicines and Medical

MCAZ Laboratory Background • It is the National quality Control laboratory(NQCL) set up to assist in the public health protection of Zimbabwe. • The QC lab provides a service and it is like a manufacturing unit whose ‘products’ include test results(COA), advice and investigations. • Scope : Quality control testing of pharmaceutical products and allied substances for quality, safety and efficacy Protecting Your Right to Quality Medicines and Medical

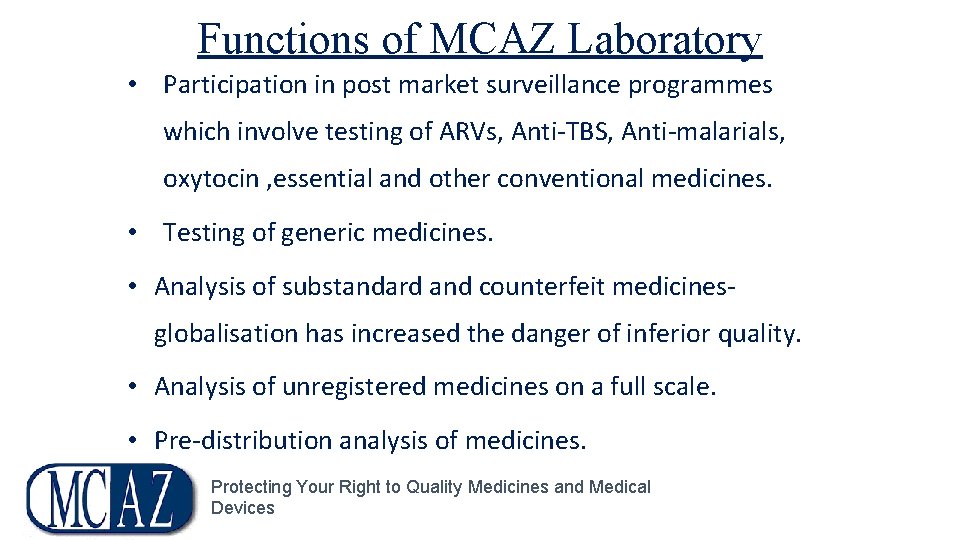
Functions of MCAZ Laboratory • Participation in post market surveillance programmes which involve testing of ARVs, Anti-TBS, Anti-malarials, oxytocin , essential and other conventional medicines. • Testing of generic medicines. • Analysis of substandard and counterfeit medicinesglobalisation has increased the danger of inferior quality. • Analysis of unregistered medicines on a full scale. • Pre-distribution analysis of medicines. Protecting Your Right to Quality Medicines and Medical Devices

Functions of MCAZ Laboratory- cont’d • Support in quality evaluation of samples for registration • Support of pharmacovigilence assessments after an adverse event on the market. • Quality testing of medicines independent of the manufacturer in relation to the safety of the human patient and/or animals. Protecting Your Right to Quality Medicines and Medical Devices

Equipment • Housed in the laboratory are the following pieces of equipment: Four(4) High Performance Liquid Chromatographs, Two(2) UV-Vis spectrophotometers, two(2) dissolution units, Millipore water purifier, a polarimeter, melting point apparatus, moisture analyser, disintegrator, p. H meter, FT-IR spectrophotometer , an AA spectrometer three (3) analytical balances, sonicators, centrifuge and a top pan balance. Protecting Your Right to Quality Medicines and Medical Devices



RCORE BACKGROUND • RCORE Defined. The RCORE would be an institution or partnership of institutions with specific regulatory science expertise as well as training capabilities: • NMRA, University faculty, National/Regional Training Centre, Scientific/Research institution, Quality Control laboratory, Pharmacovigilence centre and/or Drug information centre. • • The establishment of RCOREs is an initiative to support regulatory workforce capacity development in African Medicines Regulatory Harmonization (AMRH) Programme roundtable discussion on regulatory capacity development in Africa held in Arusha Tanzania on 30 th March 2012, made the following recommendations: • Institutionalization of regulatory training programmes in existing institutions in Africa • Establishment of Technical Working Group (TWG) on Regulatory Capacity Development in Africa Protecting Your Right to Quality Medicines and Medical Devices

RCORE BACKGROUND • Ensure sustainability of regulatory training programmes • Establishment of Regional Centres of Regulatory Excellence (RCOREs) • Regulatory training curriculum review & harmonization • The main mission of the RCOREs is to produce regulatory workforce in Africa by: • Providing academic and technical training in regulatory science applicable to different regulatory functions and managerial aspects. Protecting Your Right to Quality Medicines and Medical Devices

RCORE BACKGROUND • Allowing skills enhancement through hands-on training, twinning and exchange programmes among regulatory authorities. • Availing practical training through pharmaceutical placement in industries. • Structured competence assessment and certification(future). • Ensure sustainability of regulatory training programmes through institutionalised and structured formal curricula(future): • Protecting Your Right to Quality Medicines and Medical Devicesregulatory workforce to facilitate Increased quality review

Eligibility criteria • Regulatory capability : Technical leadership and capability to accomplish its assigned mission in at least one category of regulatory functions identified in an outstanding manner • Training capacity: An RCORE must have adequate technical personnel to undertake the regulatory functions and/or training in one of the identified categories. This will constitute a critical mass of regulatory expertise in the defined category of expertise • Governance & Management systems: The institution or partners constituting an RCORE must have appropriately authorized and functional organizational structure with proven governance and support structures • Infrastructure: An RCORE must have the necessary financial, administrative and physical resources to deliver their mandate or functions. This includes sufficient human resources in other support functions such as management, finance and legal capacity that can provide good administrative support Protecting Your Right to Quality Medicines and Medical Devices

SELECTION PROCESS • APPLICATION: Call for bids and submission of expression of interest. • ASSESSMENT: Review of received applications • APPROVAL & SELECTION: Based on the eligibility criteria • PERFORMANCE EVALUATION/REVIEW: monitoring for continued performance Protecting Your Right to Quality Medicines and Medical Devices

MCAZ ROLE AS RCORE QA/QC • Chemistry Laboratory is ISO 17025 accredited(SADCAS) and is also WHO prequalified • Chemistry aims to strengthen collaboration with other regional NRAs which do not have quality control laboratories providing quality control services, checking for quality of their medicines; Samples from Zambia, Lesotho, Burundi, Congo Brazzaville, Mali, South Sudan, Djibouti and Uzbekistan • Training on quality management system implementation- ISO 17025 – Seychelles, Armenia, Botswana and Zambia, Mozambique. • Hands on training on pharmaceutical analysis of Laboratory analysts from regional national regulatory laboratories. • Assistance in setting up national testing facilities • Strengthening of collaboration with the academia for long term training purposes. Currently working with school of Pharmacy and ICHE Protecting Your Right to Quality Medicines and Medical Devices

MCAZ ROLE AS RCORE QA/QC • • • Benefits of RCORE Status Strengthening of collaboration with the academia for long term training purposes. Providing quality control services for the regional national quality control laboratories. Hands on training on pharmaceutical analysis for laboratory analysts in the continent QMS Audits of NRAs Assist NRAs to achieve prequalification. Protecting Your Right to Quality Medicines and Medical Devices

MCAZ ROLE AS RCORE CHALLENGES • Separation of pharmaceutical analysis and training activities • Construction of a proper training centre • Equipment and staff for training purposes • Overwhelming requests for training, exchange programs and pharmaceutical analysis- capacity challenges. Protecting Your Right to Quality Medicines and Medical Devices

MCAZ ROLE AS RCORE QA/QC • Scientist exchange programmes: ZAMRA, TFDA BOTSWANA NRA and Mozambique NRA. • A handbook designed for Certificate program(short courses) and fellowship Program in regulatory science is already in place to be uploaded soon. • An advisory board to be appointed soon. • ICHE, Chemistry and school of Pharmacy have already been consulted as partners Protecting Your Right to Quality Medicines and Medical Devices

MCAZ SCIENTIST EXCHANGE PROGRAMME Protecting Your Right to Quality Medicines and Medical Devices

MCAZ ROLE AS RCORE QA/QC Protecting Your Right to Quality Medicines and Medical Devices

MCAZ ROLE AS RCORE QA/QC Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

Questions Protecting Your Right to Quality Medicines and Medical Devices

Questions Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

Protecting Your Right to Quality Medicines and Medical Devices

THANK YOU Protecting Your Right to Quality Medicines and Medical Devices

Questions Protecting Your Right to Quality Medicines and Medical Devices