The Role of Magmatic Volatiles in Arc Magmas







- Slides: 49

The Role of Magmatic Volatiles in Arc Magmas Paul Wallace University of Oregon

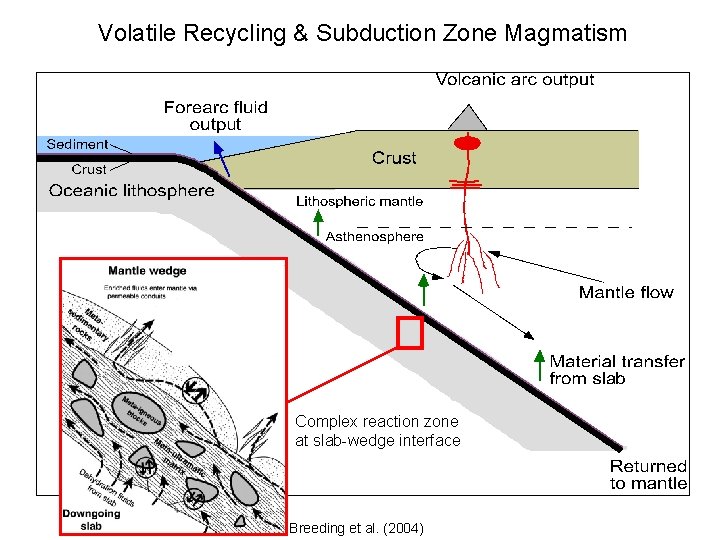
Volatile Recycling & Subduction Zone Magmatism Components in downgoing slab • Sediment • Altered oceanic crust • Serpentinized upper mantle (? ) Complex reaction zone at slab-wedge interface Breeding et al. (2004)

Outline • How do we measure magmatic volatile concentrations? • Review of experimental studies of volatile solubility • Volatile contents of basaltic arc magmas based on melt inclusion data • A comparison of volatile inputs and outputs in subduction zones • Effect of H 2 O on melting of the mantle wedge, and a brief look at how fluids and melts move through the wedge.

Problem of Magma Degassing • Solubility of volatiles is pressure dependent • Volatiles are degassed both during eruption & at depth before eruption • Bulk analysis of rock & tephra are not very useful!

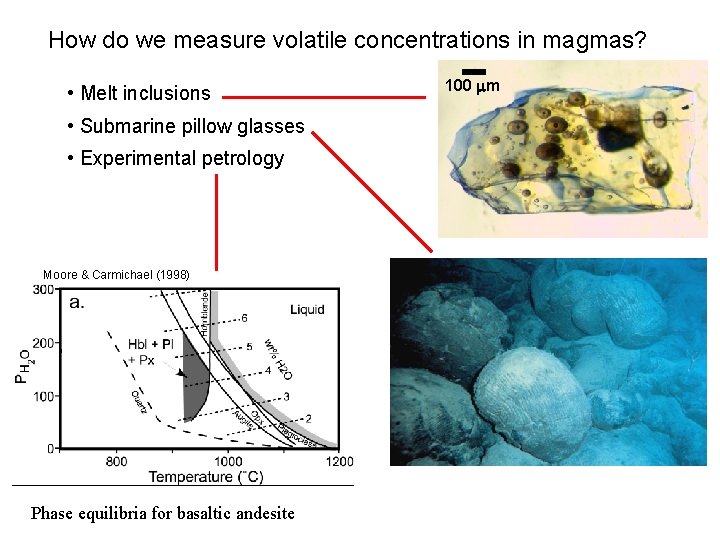
How do we measure volatile concentrations in magmas? • Melt inclusions • Submarine pillow glasses • Experimental petrology Moore & Carmichael (1998) Phase equilibria for basaltic andesite 100 mm

How do we analyze glasses & melt inclusions for volatiles? • Secondary ion mass spectrometry (SIMS or ion microprobe) H 2 O, CO 2, S, Cl, F • Fourier Transform Infrared (FTIR) spectroscopy H 2 O, CO 2 • Electron microprobe Cl, S, F • Nuclear microprobe CO 2 • Larger chips of glass from pillow rims or experimental charges can be analyzed for H 2 O and CO 2 using bulk extraction techniques e. g. , Karl-Fischer titration, manometry

What are melt inclusions & how do they form? • Primary melt inclusions form in crystals when some process interferes with the growth of a perfect crystal, causing a small volume of melt to become encased in the growing crystal. • This can occur from a variety of mechanisms, including: 1. skeletal or other irregular growth forms due to strong undercooling or non-uniform supply of nutrients 2. formation of reentrants by resorption followed by additional crystallization 3. wetting of the crystal by an immiscible phase (e. g. sulfide melt or vapor bubble) or attachment of another small crystal (e. g. spinel on olivine) resulting in irregular crystal growth and entrapment of that phase along with silicate melt • Melt inclusions can be affected by many post-entrapment processes: 1. Crystallization along the inclusion-host interface 2. Formation of a shrinkage bubble caused by cooling, which depletes the included melt in CO 2.

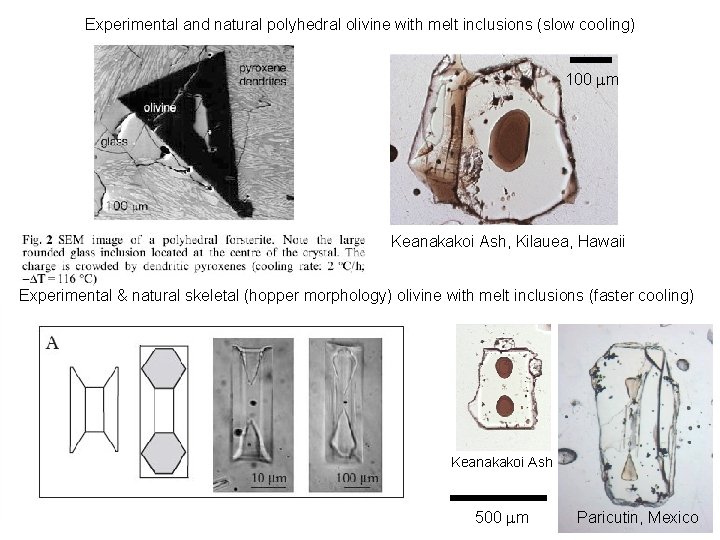
Experimental and natural polyhedral olivine with melt inclusions (slow cooling) 100 mm Keanakakoi Ash, Kilauea, Hawaii Experimental & natural skeletal (hopper morphology) olivine with melt inclusions (faster cooling) Keanakakoi Ash 500 mm Paricutin, Mexico

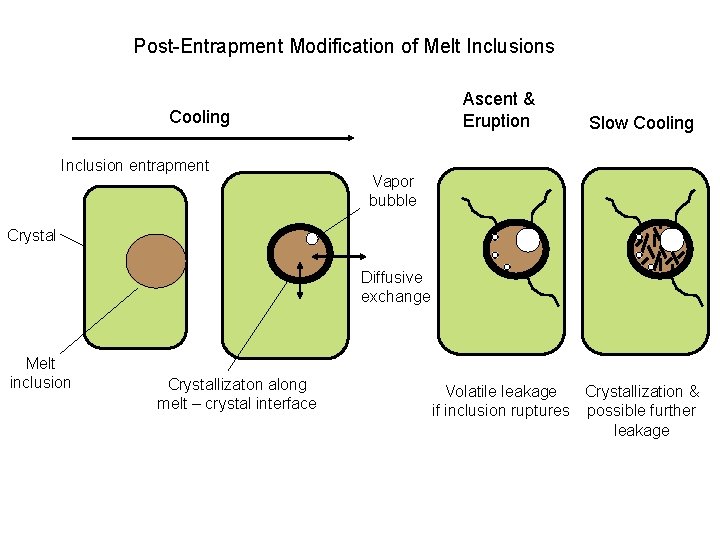
Post-Entrapment Modification of Melt Inclusions Ascent & Eruption Cooling Inclusion entrapment Slow Cooling Vapor bubble Crystal Diffusive exchange Melt inclusion Crystallizaton along melt – crystal interface Volatile leakage Crystallization & if inclusion ruptures possible further leakage

Volcanic gases - another way to get information on volatiles • Ground & airborne remote sensing • Satellite-based remote sensing • Direct sampling & analysis TOMS data for El Chichon & Pinatubo Sampling gases at Cerro Negro COSPEC at Masaya

Review of Experimentally Measured Solubilities for Volatiles Some key things to remember: • Volatile components occur as dissolved species in silicate melts, but they can also be present in an exsolved vapor phase if a melt is vapor saturated. • In laboratory experiments, it is possible to saturate melts with a nearly pure vapor phase (e. g. , H 2 O saturated), though the vapor always contains at least a small amount of dissolved solute. • In natural systems, however, multiple volatile components are always present (H 2 O, CO 2, S, Cl, F, plus less abundant volatiles like noble gases). • When the sum of the partial pressures of all dissolved volatiles in a silicate melt equals the confining pressure, the melt becomes saturated with a multicomponent (C-O-H-S-Cl-F-noble gases, etc. ) vapor phase. • Referring to natural magmas as being H 2 O saturated or CO 2 saturated is, strictly speaking, incorrect because the vapor phase is never pure and always contains more than one volatile component.

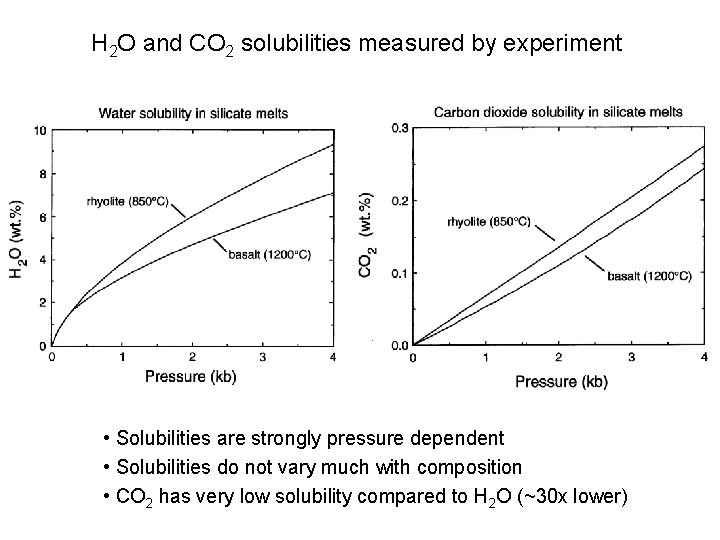
H 2 O and CO 2 solubilities measured by experiment • Solubilities are strongly pressure dependent • Solubilities do not vary much with composition • CO 2 has very low solubility compared to H 2 O (~30 x lower)

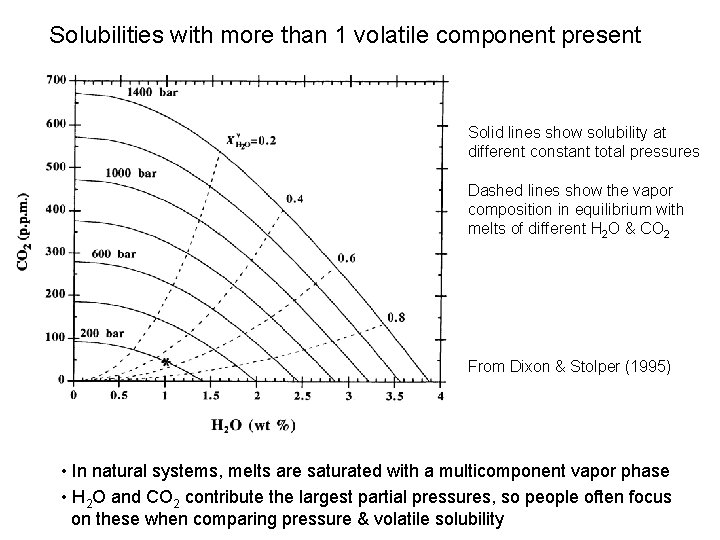
Solubilities with more than 1 volatile component present Solid lines show solubility at different constant total pressures Dashed lines show the vapor composition in equilibrium with melts of different H 2 O & CO 2 From Dixon & Stolper (1995) • In natural systems, melts are saturated with a multicomponent vapor phase • H 2 O and CO 2 contribute the largest partial pressures, so people often focus on these when comparing pressure & volatile solubility

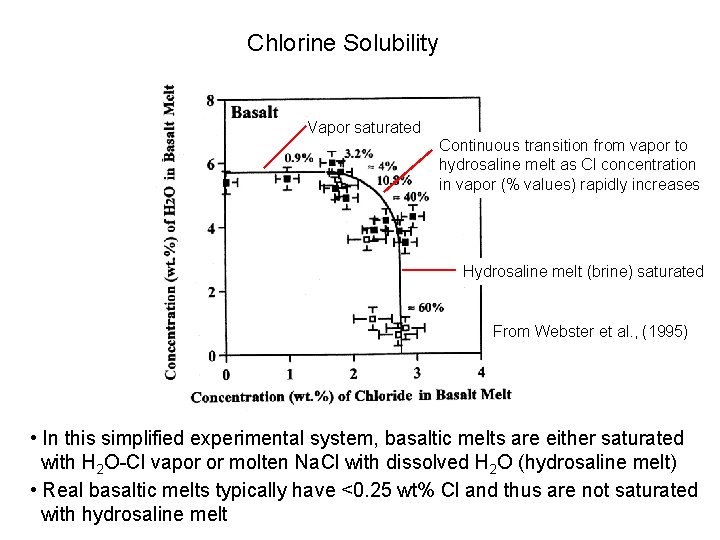
Chlorine Solubility Vapor saturated Continuous transition from vapor to hydrosaline melt as Cl concentration in vapor (% values) rapidly increases Hydrosaline melt (brine) saturated From Webster et al. , (1995) • In this simplified experimental system, basaltic melts are either saturated with H 2 O-Cl vapor or molten Na. Cl with dissolved H 2 O (hydrosaline melt) • Real basaltic melts typically have <0. 25 wt% Cl and thus are not saturated with hydrosaline melt

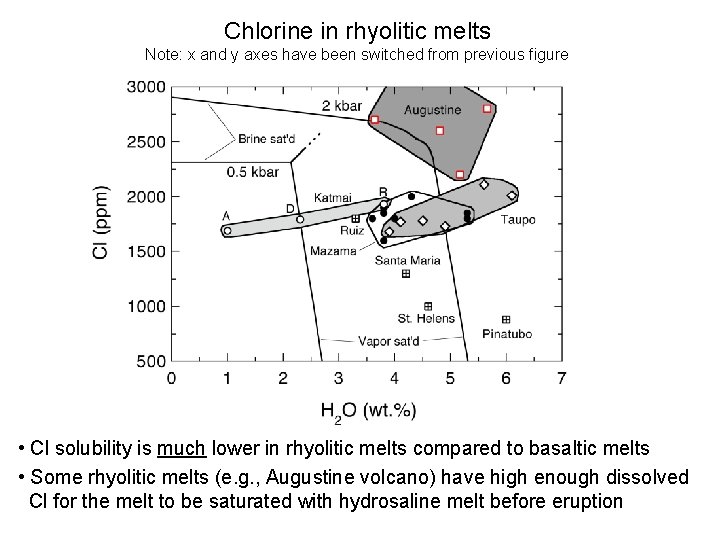
Chlorine in rhyolitic melts Note: x and y axes have been switched from previous figure • Cl solubility is much lower in rhyolitic melts compared to basaltic melts • Some rhyolitic melts (e. g. , Augustine volcano) have high enough dissolved Cl for the melt to be saturated with hydrosaline melt before eruption

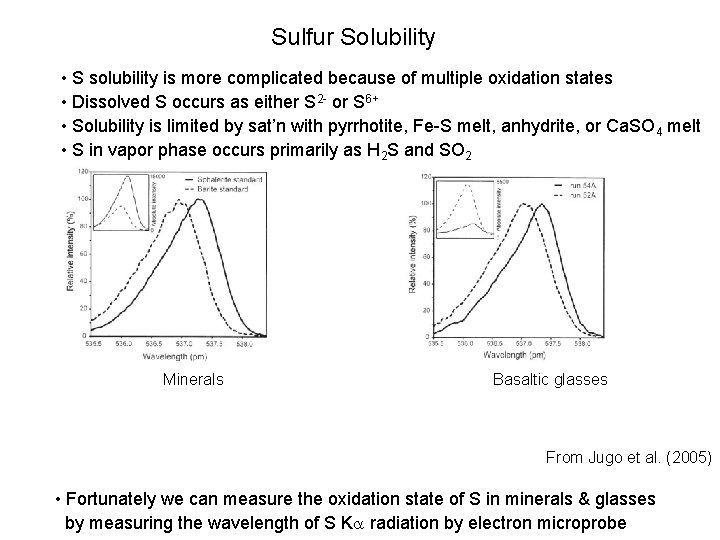
Sulfur Solubility • S solubility is more complicated because of multiple oxidation states • Dissolved S occurs as either S 2 - or S 6+ • Solubility is limited by sat’n with pyrrhotite, Fe-S melt, anhydrite, or Ca. SO 4 melt • S in vapor phase occurs primarily as H 2 S and SO 2 Minerals Basaltic glasses From Jugo et al. (2005) • Fortunately we can measure the oxidation state of S in minerals & glasses by measuring the wavelength of S K radiation by electron microprobe

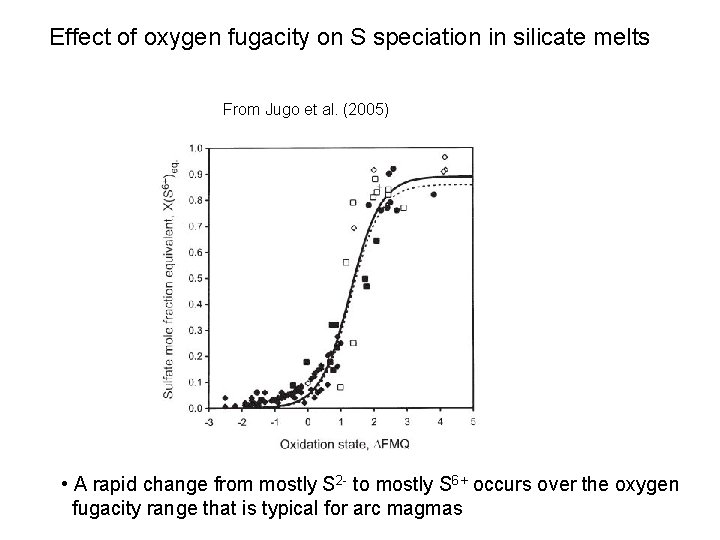
Effect of oxygen fugacity on S speciation in silicate melts From Jugo et al. (2005) • A rapid change from mostly S 2 - to mostly S 6+ occurs over the oxygen fugacity range that is typical for arc magmas

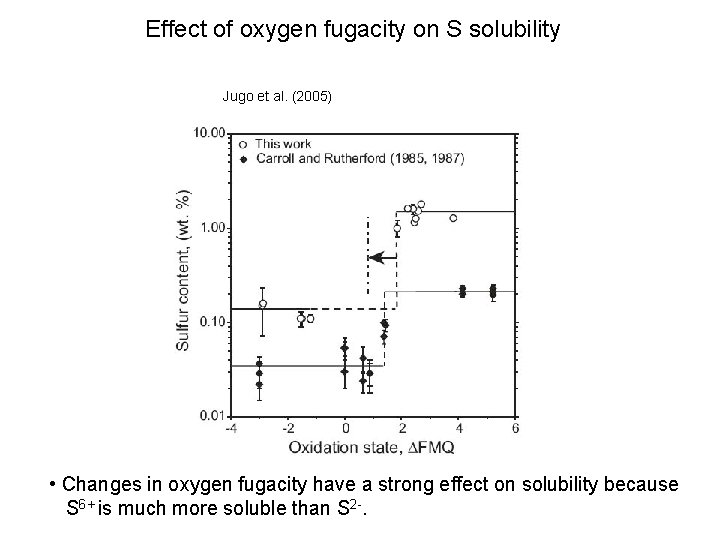
Effect of oxygen fugacity on S solubility Jugo et al. (2005) • Changes in oxygen fugacity have a strong effect on solubility because S 6+ is much more soluble than S 2 -.

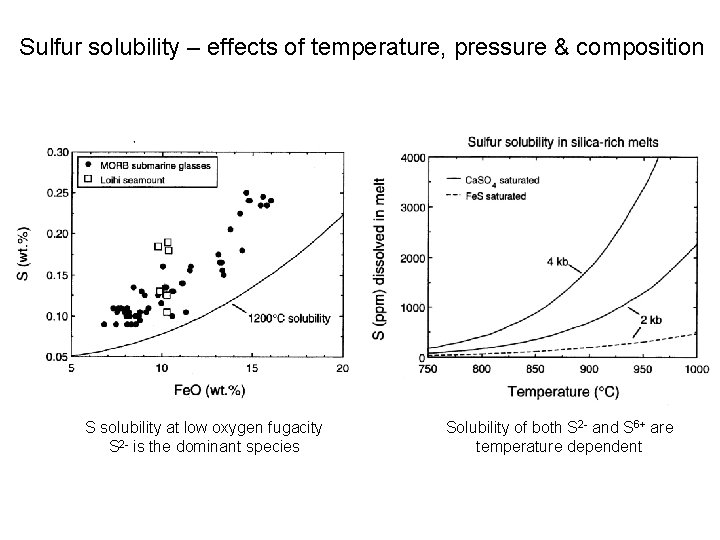
Sulfur solubility – effects of temperature, pressure & composition S solubility at low oxygen fugacity S 2 - is the dominant species Solubility of both S 2 - and S 6+ are temperature dependent

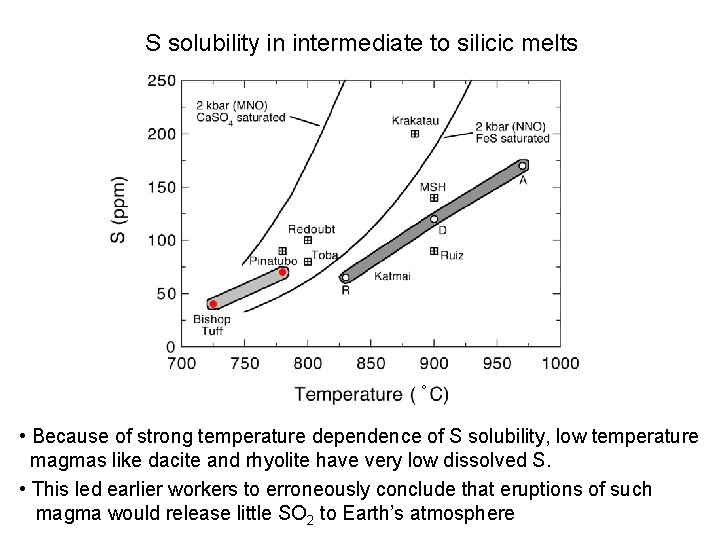
S solubility in intermediate to silicic melts ° • Because of strong temperature dependence of S solubility, low temperature magmas like dacite and rhyolite have very low dissolved S. • This led earlier workers to erroneously conclude that eruptions of such magma would release little SO 2 to Earth’s atmosphere

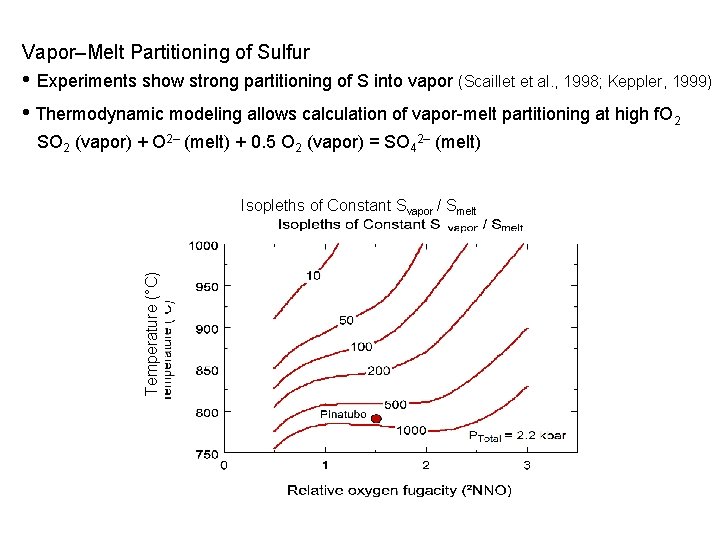
Vapor–Melt Partitioning of Sulfur • Experiments show strong partitioning of S into vapor (Scaillet et al. , 1998; Keppler, 1999) • Thermodynamic modeling allows calculation of vapor-melt partitioning at high f. O 2 SO 2 (vapor) + O 2– (melt) + 0. 5 O 2 (vapor) = SO 42– (melt) Temperature (°C) Isopleths of Constant Svapor / Smelt

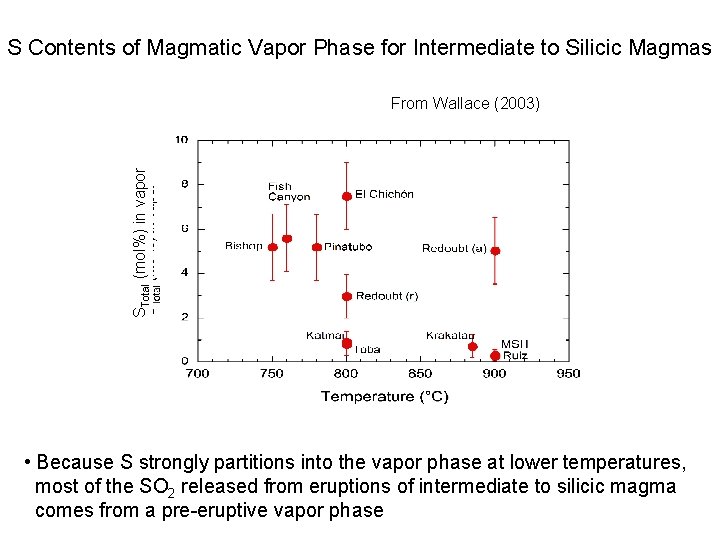
S Contents of Magmatic Vapor Phase for Intermediate to Silicic Magmas STotal (mol%) in vapor From Wallace (2003) • Because S strongly partitions into the vapor phase at lower temperatures, most of the SO 2 released from eruptions of intermediate to silicic magma comes from a pre-eruptive vapor phase

What can melt inclusions tell us about volatiles if magmas are generally vapor saturated? • Only part of the story – melt inclusions tell us the concentrations of dissolved volatiles • Information captured by melt inclusions depends on the vapor / melt partition coefficient, and thus is different for each volatile component • Melt inclusions also provide information on magma storage depths and vapor phase compositions (e. g. , use of H 2 O vs. CO 2 diagram) • Diagrams in the next two figures show much of the initial amount of each volatile is still dissolved at the time inclusions are trapped

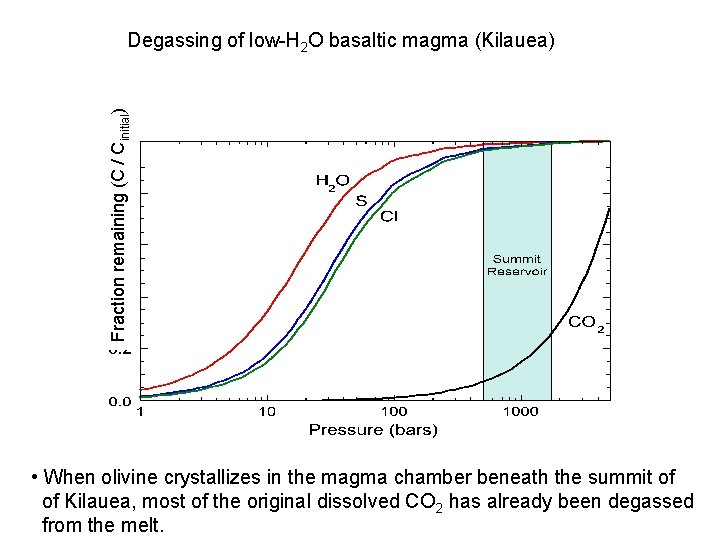
Fraction remaining (C / Cinitial) Degassing of low-H 2 O basaltic magma (Kilauea) • When olivine crystallizes in the magma chamber beneath the summit of of Kilauea, most of the original dissolved CO 2 has already been degassed from the melt.

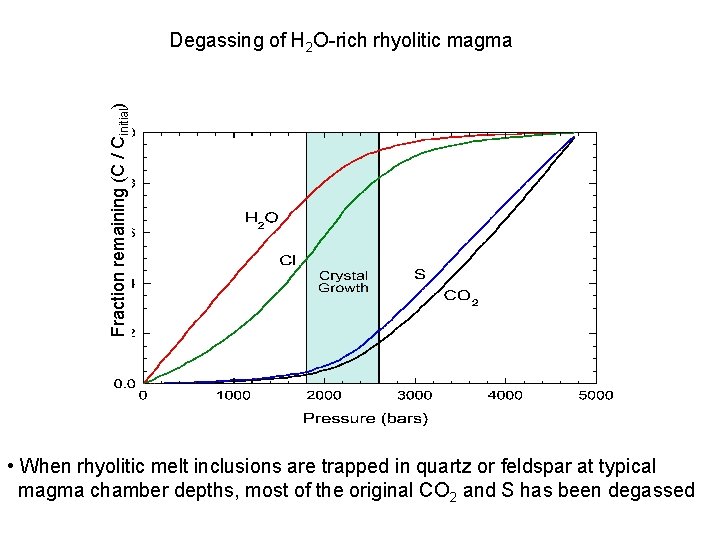
Fraction remaining (C / Cinitial) Degassing of H 2 O-rich rhyolitic magma • When rhyolitic melt inclusions are trapped in quartz or feldspar at typical magma chamber depths, most of the original CO 2 and S has been degassed

Volatile contents of mafic arc magmas based on melt inclusions 100 mm Blue Lake Maar, Oregon Cascades Jorullo volcano, Mexico Photos by Emily Johnson, Univ. of Oregon

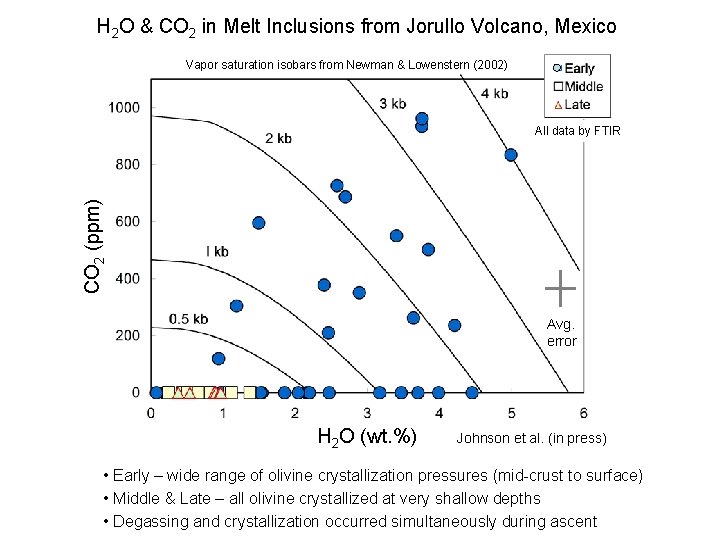
H 2 O & CO 2 in Melt Inclusions from Jorullo Volcano, Mexico Vapor saturation isobars from Newman & Lowenstern (2002) CO 2 (ppm) All data by FTIR Avg. error H 2 O (wt. %) Johnson et al. (in press) • Early – wide range of olivine crystallization pressures (mid-crust to surface) • Middle & Late – all olivine crystallized at very shallow depths • Degassing and crystallization occurred simultaneously during ascent

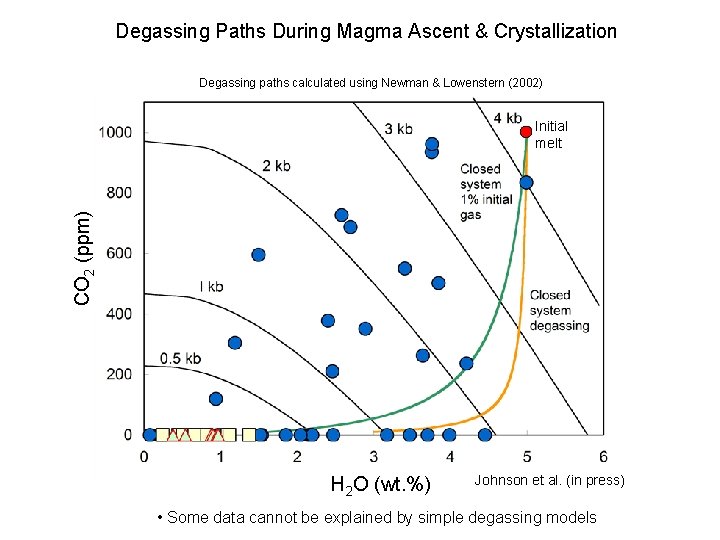
Degassing Paths During Magma Ascent & Crystallization Degassing paths calculated using Newman & Lowenstern (2002) CO 2 (ppm) Initial melt H 2 O (wt. %) Johnson et al. (in press) • Some data cannot be explained by simple degassing models

Effects of degassing • Melt inclusion data from a single volcano or even a single eruptive unit often show a range of H 2 O and CO 2 values. • In most cases, this range reflects variable degassing during ascent before the melts were trapped in growing olivine crystals. • S can also be affected by this variable degassing, but Cl and F solubilities are so high that they tend to stay dissolved in the melt. • From a large number of analyzed melt inclusions (preferably 15 -25), the highest analyzed volatile values provide a minimum estimate of the primary volatile content of the melt before any degassing. • The data shown on the following slides are for the least degassed melt inclusions from a number of different volcanoes.

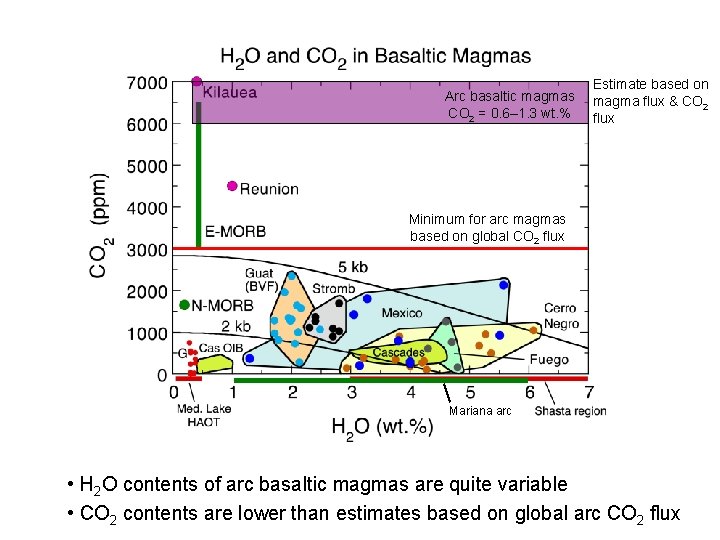
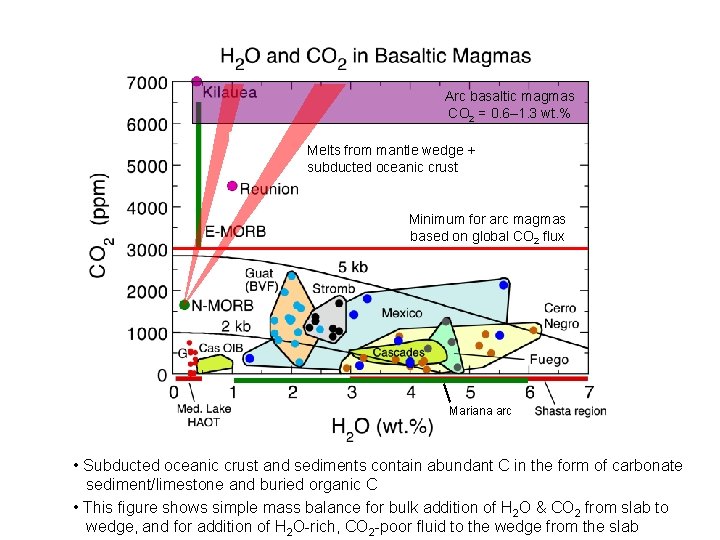
Arc basaltic magmas CO 2 = 0. 6– 1. 3 wt. % Estimate based on magma flux & CO 2 flux Minimum for arc magmas based on global CO 2 flux Mariana arc • H 2 O contents of arc basaltic magmas are quite variable • CO 2 contents are lower than estimates based on global arc CO 2 flux

Arc basaltic magmas CO 2 = 0. 6– 1. 3 wt. % Melts from mantle wedge + subducted sediment subducted oceanic crust Minimum for arc magmas based on global CO 2 flux Mariana arc • Subducted oceanic crust and sediments contain abundant C in the form of carbonate sediment/limestone and buried organic C • This figure shows simple mass balance for bulk addition of H 2 O & CO 2 from slab to wedge, and for addition of H 2 O-rich, CO 2 -poor fluid to the wedge from the slab

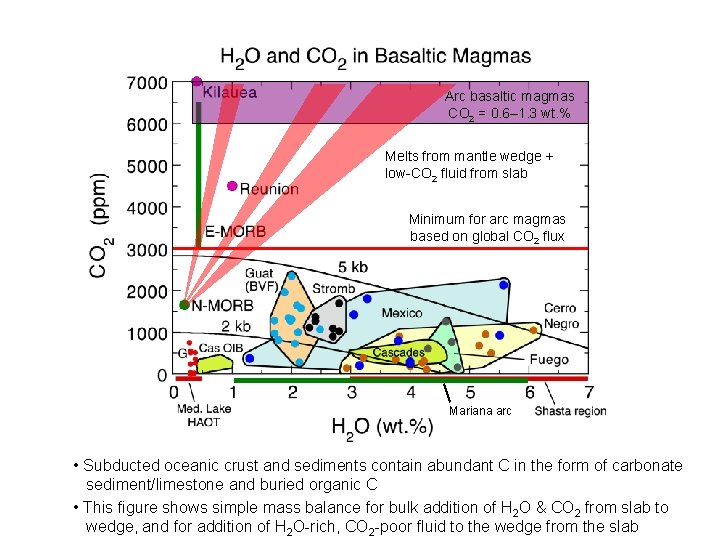
Arc basaltic magmas CO 2 = 0. 6– 1. 3 wt. % Melts from mantle wedge + low-CO 2 fluid from slab Minimum for arc magmas based on global CO 2 flux Mariana arc • Subducted oceanic crust and sediments contain abundant C in the form of carbonate sediment/limestone and buried organic C • This figure shows simple mass balance for bulk addition of H 2 O & CO 2 from slab to wedge, and for addition of H 2 O-rich, CO 2 -poor fluid to the wedge from the slab

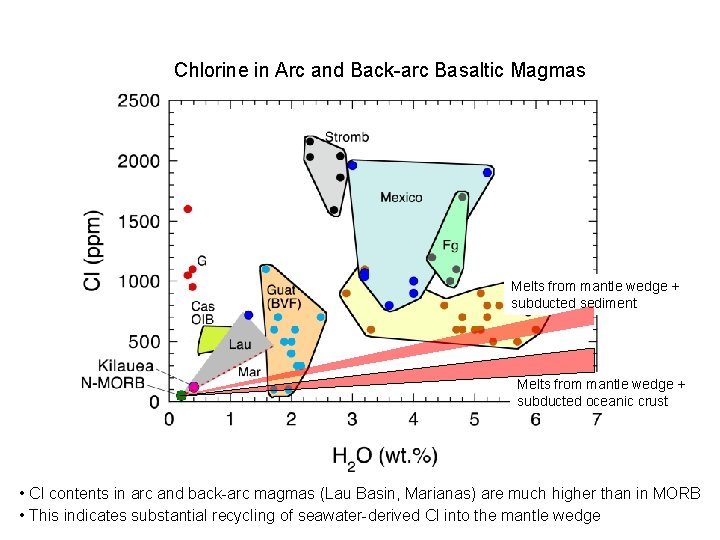
Chlorine in Arc and Back-arc Basaltic Magmas Melts from mantle wedge + subducted sediment Melts from mantle wedge + subducted oceanic crust • Cl contents in arc and back-arc magmas (Lau Basin, Marianas) are much higher than in MORB • This indicates substantial recycling of seawater-derived Cl into the mantle wedge

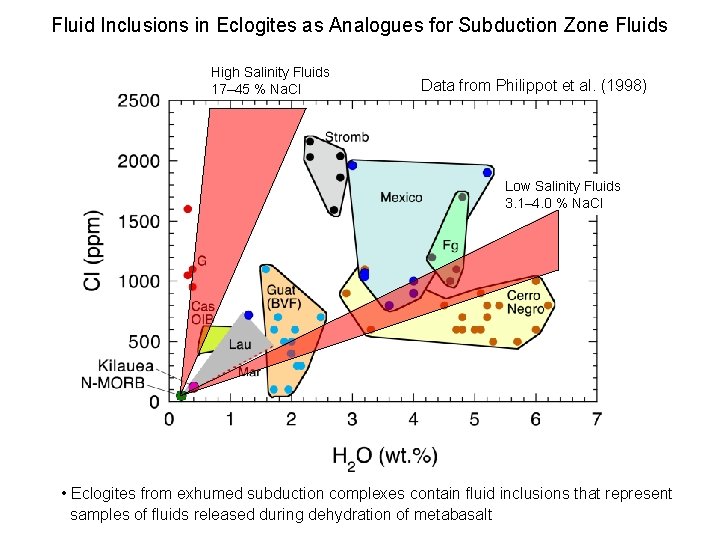
Fluid Inclusions in Eclogites as Analogues for Subduction Zone Fluids High Salinity Fluids 17– 45 % Na. Cl Data from Philippot et al. (1998) Low Salinity Fluids 3. 1– 4. 0 % Na. Cl • Eclogites from exhumed subduction complexes contain fluid inclusions that represent samples of fluids released during dehydration of metabasalt

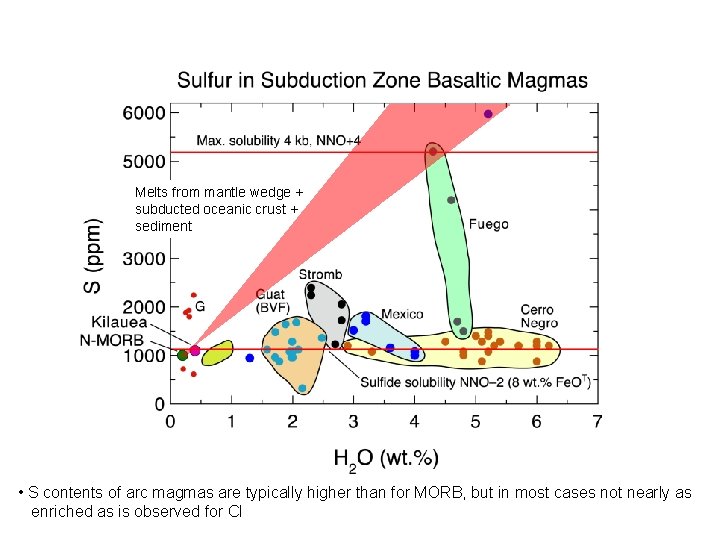
Melts from mantle wedge + subducted oceanic crust + sediment • S contents of arc magmas are typically higher than for MORB, but in most cases not nearly as enriched as is observed for Cl

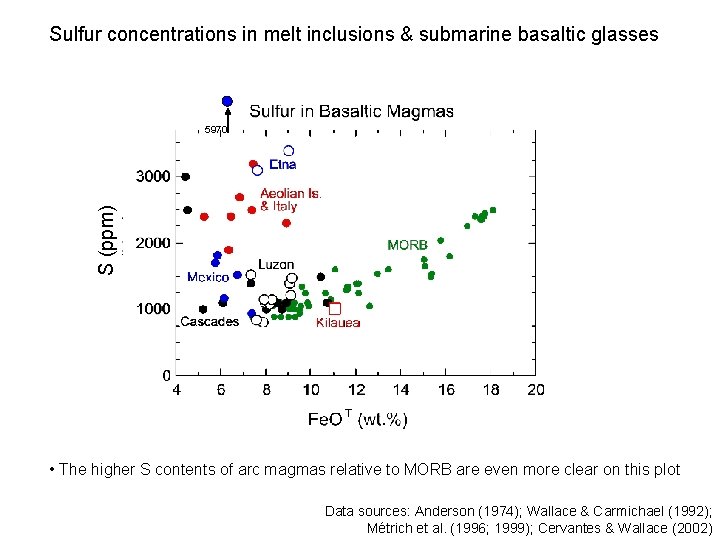
Sulfur concentrations in melt inclusions & submarine basaltic glasses S (ppm) 5970 • The higher S contents of arc magmas relative to MORB are even more clear on this plot Data sources: Anderson (1974); Wallace & Carmichael (1992); Métrich et al. (1996; 1999); Cervantes & Wallace (2002)

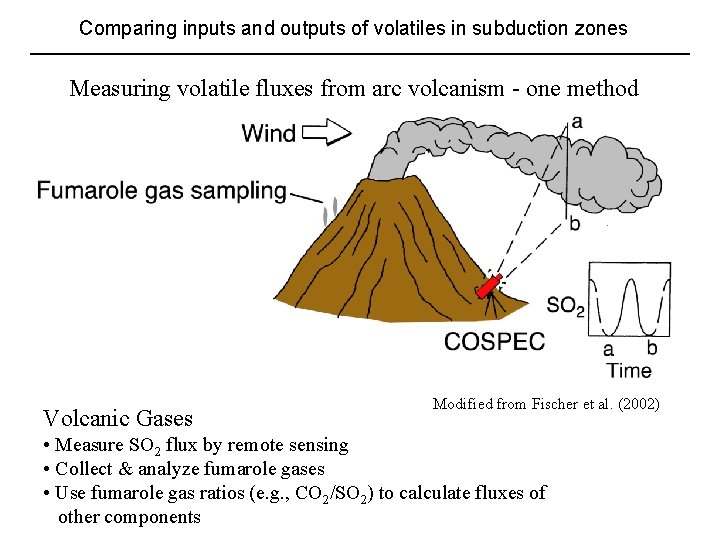
Comparing inputs and outputs of volatiles in subduction zones Measuring volatile fluxes from arc volcanism - one method Volcanic Gases Modified from Fischer et al. (2002) • Measure SO 2 flux by remote sensing • Collect & analyze fumarole gases • Use fumarole gas ratios (e. g. , CO 2/SO 2) to calculate fluxes of other components

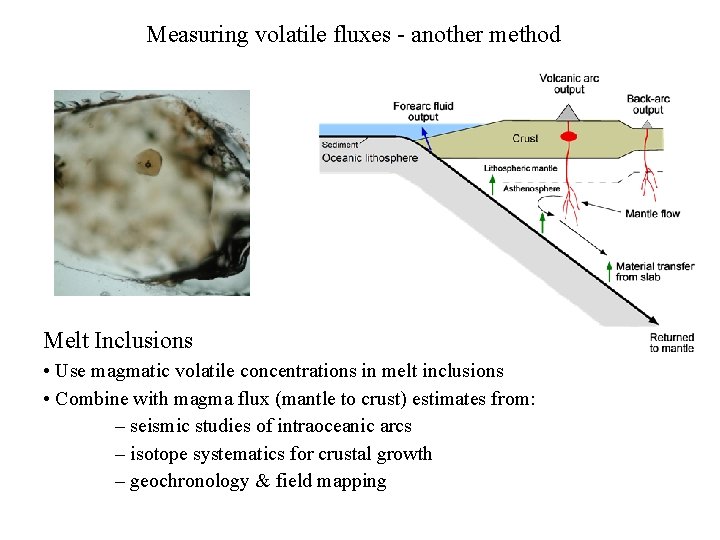
Measuring volatile fluxes - another method Melt Inclusions • Use magmatic volatile concentrations in melt inclusions • Combine with magma flux (mantle to crust) estimates from: – seismic studies of intraoceanic arcs – isotope systematics for crustal growth – geochronology & field mapping

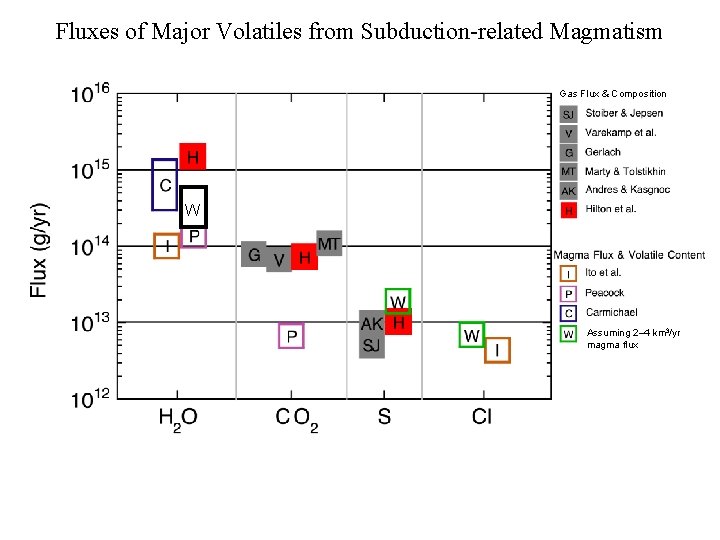
Fluxes of Major Volatiles from Subduction-related Magmatism Gas Flux & Composition W Assuming 2– 4 km 3/yr magma flux

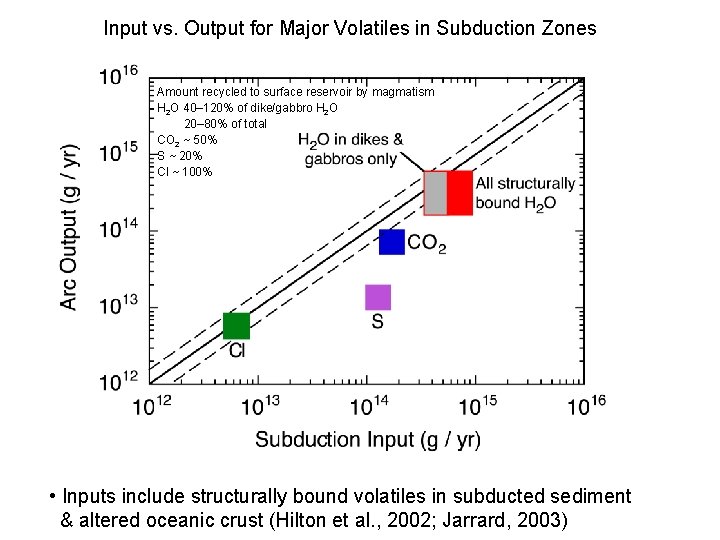
Input vs. Output for Major Volatiles in Subduction Zones Amount recycled to surface reservoir by magmatism H 2 O 40– 120% of dike/gabbro H 2 O 20– 80% of total CO 2 ~ 50% S ~ 20% Cl ~ 100% • Inputs include structurally bound volatiles in subducted sediment & altered oceanic crust (Hilton et al. , 2002; Jarrard, 2003)

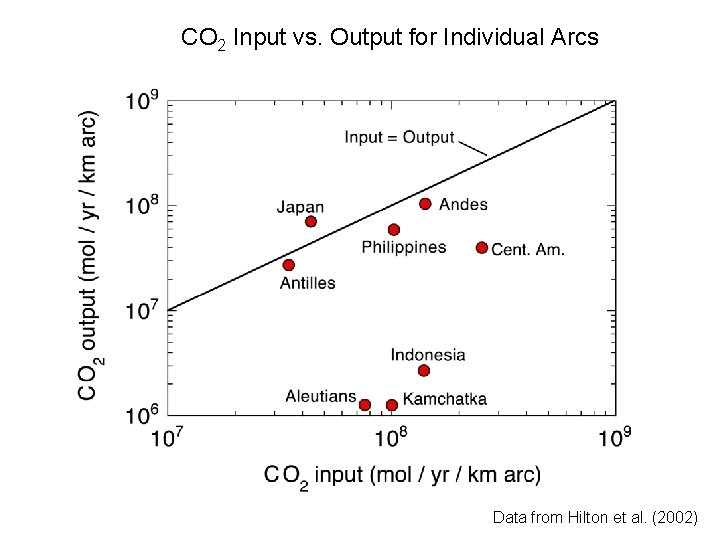
CO 2 Input vs. Output for Individual Arcs Data from Hilton et al. (2002)

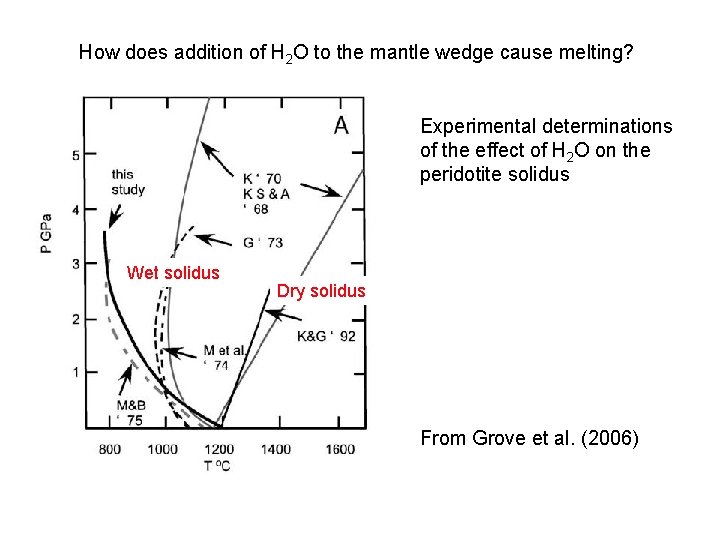
How does addition of H 2 O to the mantle wedge cause melting? Experimental determinations of the effect of H 2 O on the peridotite solidus Wet solidus Dry solidus From Grove et al. (2006)

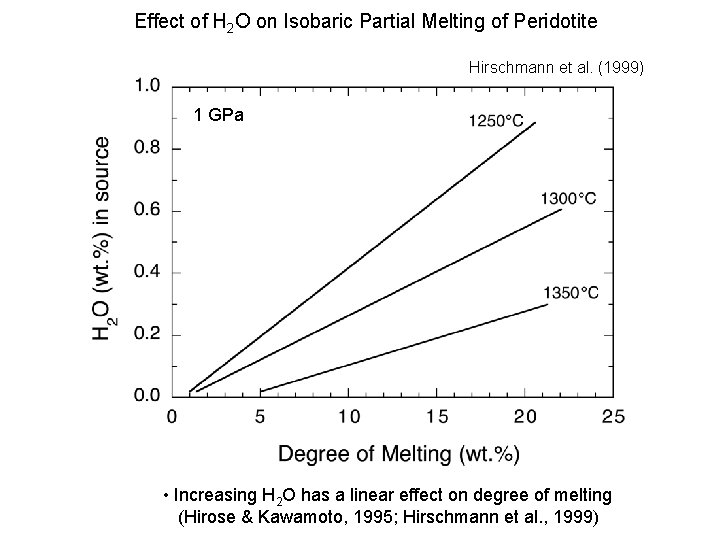
Effect of H 2 O on Isobaric Partial Melting of Peridotite Hirschmann et al. (1999) 1 GPa Xitle • Increasing H 2 O has a linear effect on degree of melting (Hirose & Kawamoto, 1995; Hirschmann et al. , 1999)

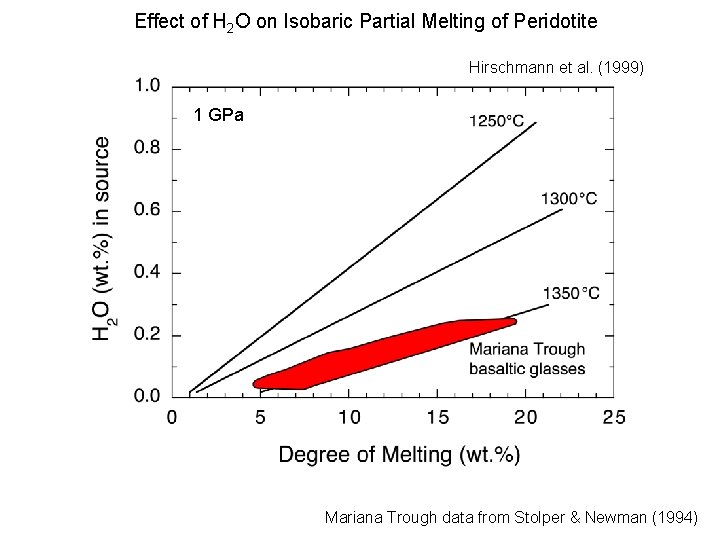
Effect of H 2 O on Isobaric Partial Melting of Peridotite Hirschmann et al. (1999) 1 GPa Mariana Trough data from Stolper & Newman (1994)

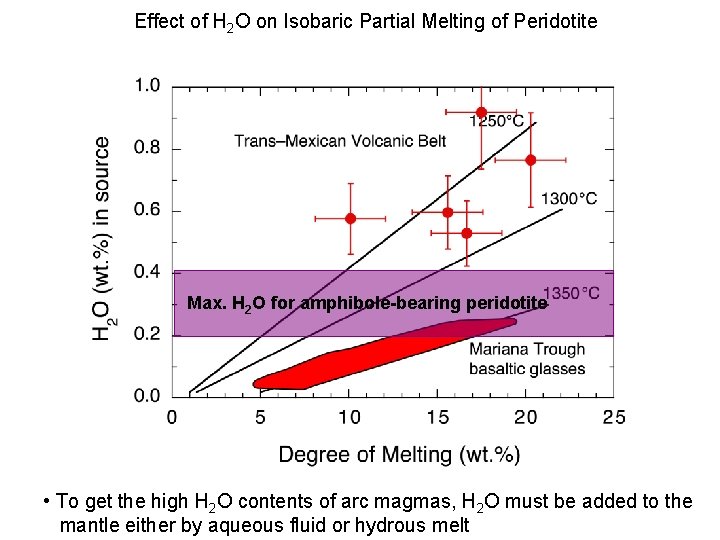
Effect of H 2 O on Isobaric Partial Melting of Peridotite Max. H 2 O for amphibole-bearing peridotite • To get the high H 2 O contents of arc magmas, H 2 O must be added to the mantle either by aqueous fluid or hydrous melt

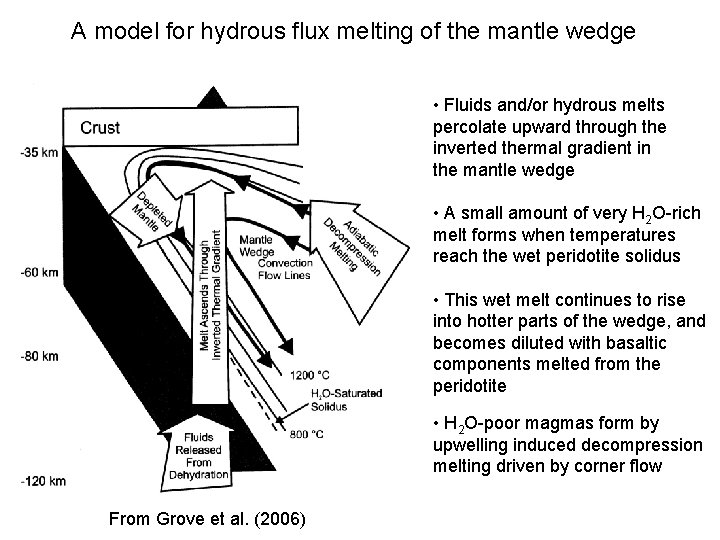
A model for hydrous flux melting of the mantle wedge • Fluids and/or hydrous melts percolate upward through the inverted thermal gradient in the mantle wedge • A small amount of very H 2 O-rich melt forms when temperatures reach the wet peridotite solidus • This wet melt continues to rise into hotter parts of the wedge, and becomes diluted with basaltic components melted from the peridotite • H 2 O-poor magmas form by upwelling induced decompression melting driven by corner flow From Grove et al. (2006)

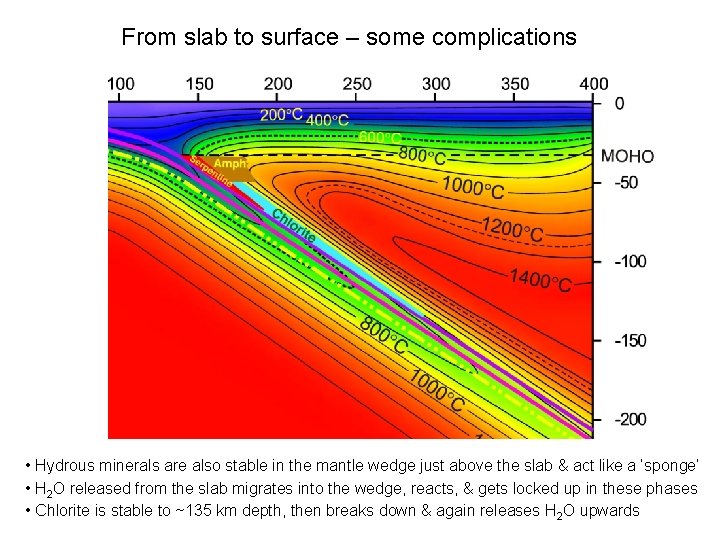
From slab to surface – some complications • Hydrous minerals are also stable in the mantle wedge just above the slab & act like a ‘sponge’ • H 2 O released from the slab migrates into the wedge, reacts, & gets locked up in these phases • Chlorite is stable to ~135 km depth, then breaks down & again releases H 2 O upwards

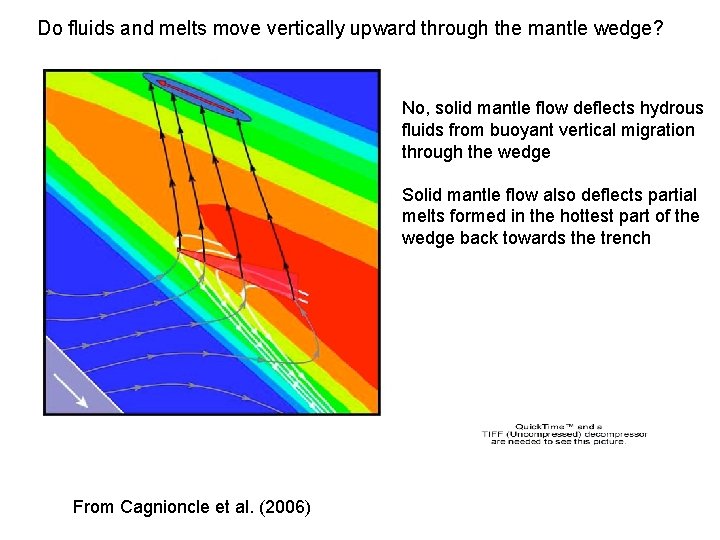
Do fluids and melts move vertically upward through the mantle wedge? No, solid mantle flow deflects hydrous fluids from buoyant vertical migration through the wedge Solid mantle flow also deflects partial melts formed in the hottest part of the wedge back towards the trench From Cagnioncle et al. (2006)

And finally, mafic arc magmas have enough H 2 O to cause explosive eruptions (violent strombolian, sub-plinian, and occasionally plinian) that produce large amounts of ash and lapilli
 Magmatic intrusion
Magmatic intrusion Poise
Poise Sales neutras
Sales neutras Csillámpala érc
Csillámpala érc Magmas dosage form
Magmas dosage form Arc promoteur vs arc investigateur
Arc promoteur vs arc investigateur Minor arc and major arc
Minor arc and major arc What is mean by minor
What is mean by minor Arc emu88 com arc info 45 100045 html
Arc emu88 com arc info 45 100045 html C# azure worker role example
C# azure worker role example Role taking krappmann
Role taking krappmann Statuses and their related roles determine the structure
Statuses and their related roles determine the structure Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Chụp tư thế worms-breton
Chụp tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Cái miệng nó xinh thế
Cái miệng nó xinh thế Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Tư thế ngồi viết
Tư thế ngồi viết Slidetodoc
Slidetodoc Giọng cùng tên là
Giọng cùng tên là Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Hát lên người ơi alleluia
Hát lên người ơi alleluia Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính thế năng
Công thức tính thế năng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Lời thề hippocrates
Lời thề hippocrates Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu điện thế nghỉ
điện thế nghỉ Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Fecboak
Fecboak Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới