THE ROLE OF IMIPENEM CILASTATIN AND RELEBACTAM CORINA
THE ROLE OF IMIPENEM, CILASTATIN, AND RELEBACTAM CORINA MONTGOMERY, PHARM. D. PGY 1 RESIDENT ERIE VA MEDICAL CENTER

DISCLOSURE • The presenter has no conflicts of interest • The views expressed in this presentation are those of the presenter and do not necessarily reflect the position or policy of the Department of Veterans Affairs of the United States government • This presentation is the result of work supported with resources and the use of facilities at the Erie VA Medical Center, Erie, PA

OBJECTIVES Pharmacist • Review background information about imipenem, cilastatin, and relebactam including mechanism of action, indications, and adverse effects • Discuss and analyze the current available literature to determine the appropriate use of imipenem, cilastatin, and relebactam Technician • Recognize the route of administration

BACKGROUND • Antibacterial agent • FDA approved July 2019 • Received FDA Qualified Infectious Disease Product designation • Intravenous administration • Manufactured by Merck
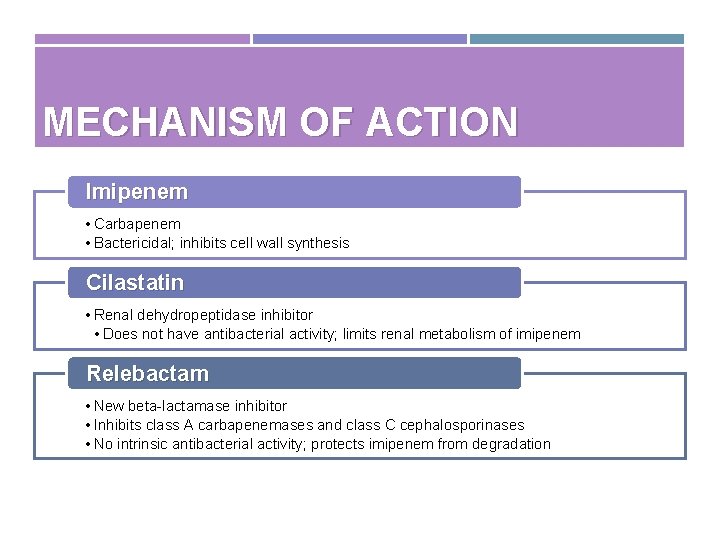
MECHANISM OF ACTION Imipenem • Carbapenem • Bactericidal; inhibits cell wall synthesis Cilastatin • Renal dehydropeptidase inhibitor • Does not have antibacterial activity; limits renal metabolism of imipenem Relebactam • New beta-lactamase inhibitor • Inhibits class A carbapenemases and class C cephalosporinases • No intrinsic antibacterial activity; protects imipenem from degradation

INDICATIONS • 18 years and older who have limited or no alternative treatment options • Complicated urinary tract infections, including pyelonephritis • Complicated intra-abdominal infections
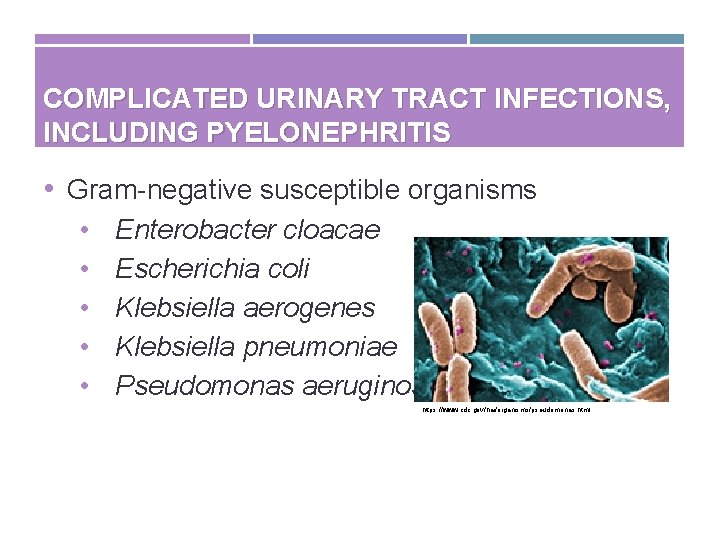
COMPLICATED URINARY TRACT INFECTIONS, INCLUDING PYELONEPHRITIS • Gram-negative susceptible organisms • • • Enterobacter cloacae Escherichia coli Klebsiella aerogenes Klebsiella pneumoniae Pseudomonas aeruginosa https: //www. cdc. gov/hai/organisms/pseudomonas. html
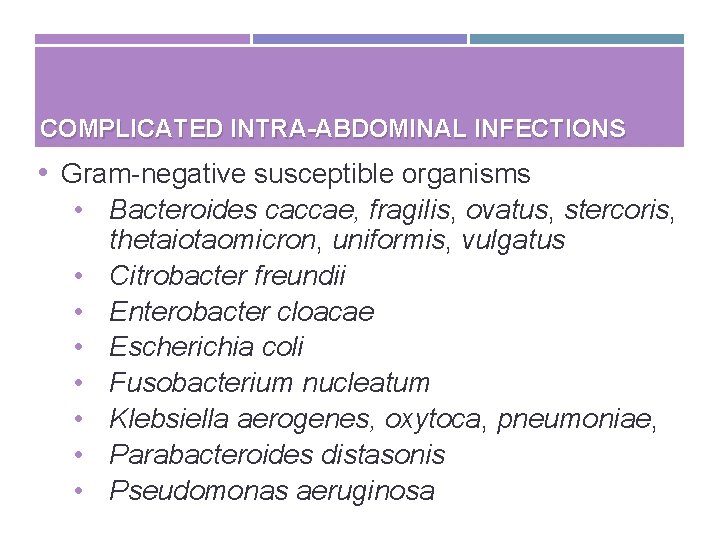
COMPLICATED INTRA-ABDOMINAL INFECTIONS • Gram-negative susceptible organisms • Bacteroides caccae, fragilis, ovatus, stercoris, thetaiotaomicron, uniformis, vulgatus • Citrobacter freundii • Enterobacter cloacae • Escherichia coli • Fusobacterium nucleatum • Klebsiella aerogenes, oxytoca, pneumoniae, • Parabacteroides distasonis • Pseudomonas aeruginosa
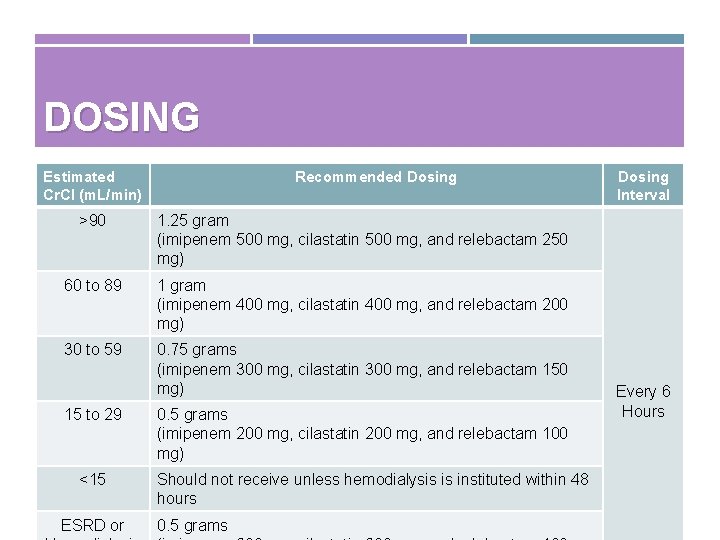
DOSING Estimated Cr. Cl (m. L/min) Recommended Dosing >90 1. 25 gram (imipenem 500 mg, cilastatin 500 mg, and relebactam 250 mg) 60 to 89 1 gram (imipenem 400 mg, cilastatin 400 mg, and relebactam 200 mg) 30 to 59 0. 75 grams (imipenem 300 mg, cilastatin 300 mg, and relebactam 150 mg) 15 to 29 <15 ESRD or 0. 5 grams (imipenem 200 mg, cilastatin 200 mg, and relebactam 100 mg) Should not receive unless hemodialysis is instituted within 48 hours 0. 5 grams Dosing Interval Every 6 Hours
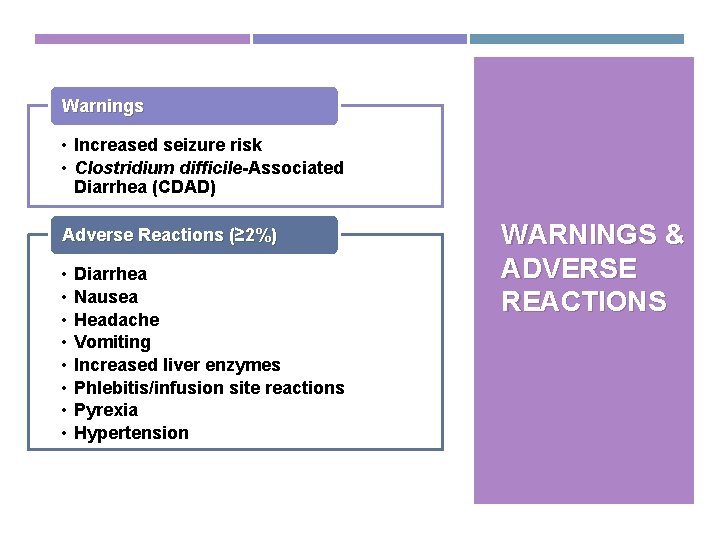
Warnings • Increased seizure risk • Clostridium difficile-Associated Diarrhea (CDAD) Adverse Reactions (≥ 2%) • • Diarrhea Nausea Headache Vomiting Increased liver enzymes Phlebitis/infusion site reactions Pyrexia Hypertension WARNINGS & ADVERSE REACTIONS
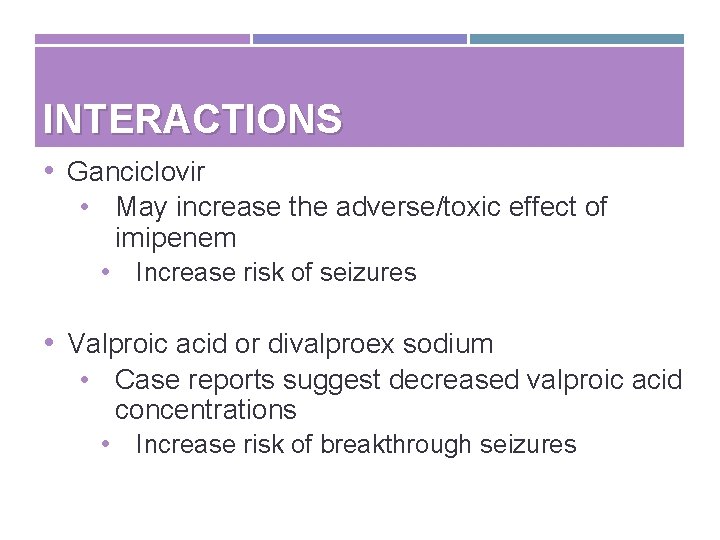
INTERACTIONS • Ganciclovir • May increase the adverse/toxic effect of imipenem • Increase risk of seizures • Valproic acid or divalproex sodium • Case reports suggest decreased valproic acid concentrations • Increase risk of breakthrough seizures
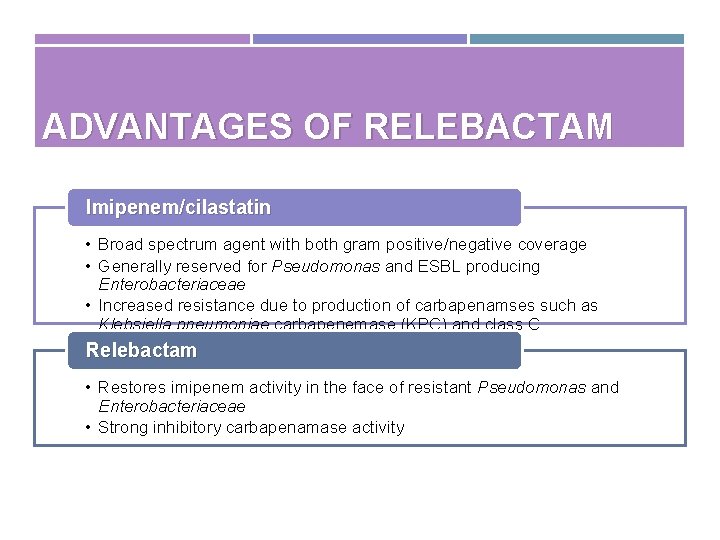
ADVANTAGES OF RELEBACTAM Imipenem/cilastatin • Broad spectrum agent with both gram positive/negative coverage • Generally reserved for Pseudomonas and ESBL producing Enterobacteriaceae • Increased resistance due to production of carbapenamses such as Klebsiella pneumoniae carbapenemase (KPC) and class C cephalosporinases (eg, Amp. C) Relebactam • Restores imipenem activity in the face of resistant Pseudomonas and Enterobacteriaceae • Strong inhibitory carbapenamase activity

RESTORE-IMI 1 Methods • Phase 3 double-blind randomized controlled trial • Compared imipenem/relebactam versus colistin+imipenem • Primary endpoint: favorable overall response • Defined by relevant endpoints for each infection

RESTORE-IMI 1 Inclusion criteria • • ≥ 18 years of age Infection types limited to HAP, VABP, c. IAI, c. UTI Suspected causative pathogens • Identified as a Gram-negative bacterium • Has culture-confirmed imipenem resistance • Has culture-confirmed susceptibility to colistin Must be physically incapable of reproduction or use at least two forms of contraception

RESTORE-IMI 1 Exclusion criteria • APACHE II score >30 at screening • Concurrent infection • Systemic colistin treatment for >24 hours within the 72 hours immediately prior to initiation of study therapy • HAP/VAP caused by an obstructive process • History of serious allergy, hypersensitivity to any of the study drug classes • Pregnant, expecting to conceive, or breastfeeding • Seizure disorder • Hemodialysis/peritoneal dialysis or Cr. Cl <15 m. L/min at screening
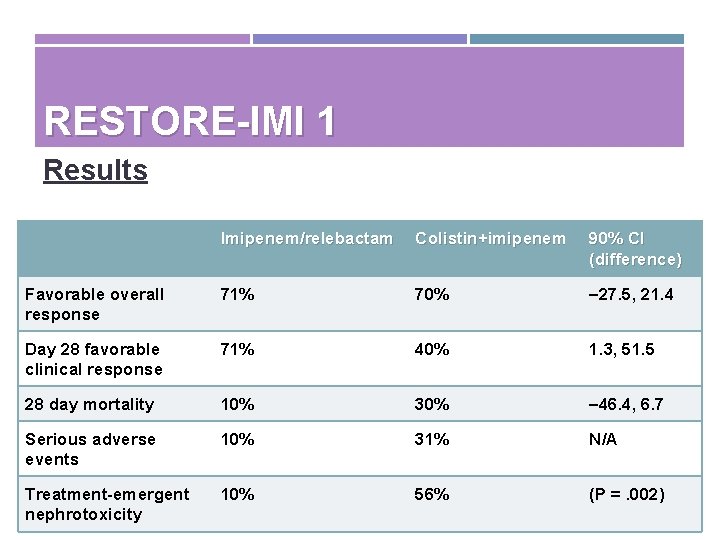
RESTORE-IMI 1 Results Imipenem/relebactam Colistin+imipenem 90% CI (difference) Favorable overall response 71% 70% – 27. 5, 21. 4 Day 28 favorable clinical response 71% 40% 1. 3, 51. 5 28 day mortality 10% 30% – 46. 4, 6. 7 Serious adverse events 10% 31% N/A Treatment-emergent nephrotoxicity 10% 56% (P =. 002)

SUMMARY • Combination of previously approved agents, imipenem/cilastatin, and a new agent, relebactam • Imipenem, cilastatin, and relebactam should be reserved for those who do not have additional options to treat: • Complicated urinary tract infections, including pyelonephritis • Complicated intra-abdominal infections
REVIEW QUESTIONS
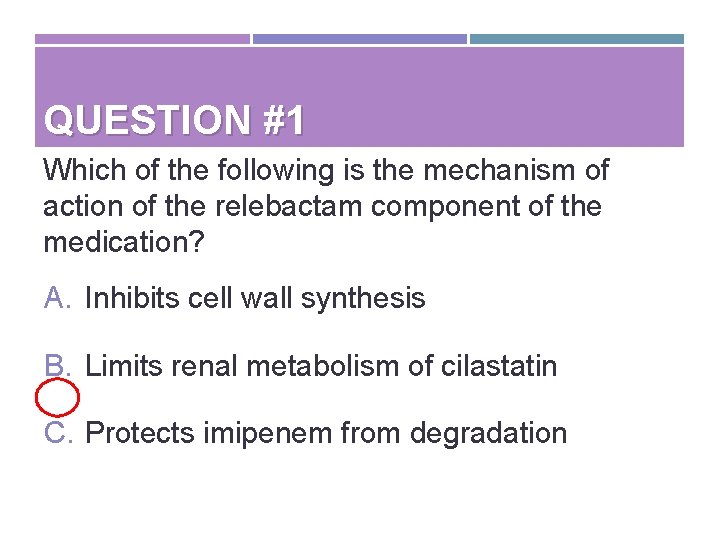
QUESTION #1 Which of the following is the mechanism of action of the relebactam component of the medication? A. Inhibits cell wall synthesis B. Limits renal metabolism of cilastatin C. Protects imipenem from degradation

QUESTION #2 What is the correct dose for a 51 year old patient with a Cr. Cl of 45 m. L/min? A. 1. 25 gram IV infusion over 30 minutes every 6 hours B. 1 gram IV infusion over 30 minutes every 6 hours C. 0. 75 grams IV infusion over 30 minutes every 6 hours D. 0. 5 grams IV infusion over 60 minutes every 8 hours
![REFERENCES • Recarbrio [package insert]. Whitehouse Station, NJ: Merck & Co. , Inc. ; REFERENCES • Recarbrio [package insert]. Whitehouse Station, NJ: Merck & Co. , Inc. ;](http://slidetodoc.com/presentation_image_h/bd3aa5c66865fbe04b20005df46dcffb/image-21.jpg)
REFERENCES • Recarbrio [package insert]. Whitehouse Station, NJ: Merck & Co. , Inc. ; 2019. • FDA approves new treatment for complicated urinary tract and complicated intra-abdominal infections [news release]. Silver Spring, MD; July 17, 2019: FDA website. https: //www. fda. gov/newsevents/press-announcements/fda-approves-new-treatment-complicated-urinary-tract-andcomplicated-intra-abdominal-infections. Accessed October 5, 2019. • FDA Approves Merck’s RECARBRIO™ (imipenem, cilastatin, and relebactam) For the Treatment of Adults with Complicated Urinary Tract and Complicated Intra-Abdominal Bacterial Infections Where Limited or No Alternative Treatment Options Are Available [news release]. Kenilworth, NJ; July 17, 2019: Merck. https: //www. mrknewsroom. com/news-release/prescription-medicine-news/fdaapproves-mercks-recarbrio-imipenem-cilastatin-and-releba. Accessed October 5, 2019. • Johann Motsch, Cláudia Murta de Oliveira, Viktor Stus, Iftihar Köksal, Olexiy Lyulko, Helen W Boucher, Keith S Kaye, Thomas M File, Michelle L Brown, Ireen Khan, Jiejun Du, Hee-Koung Joeng, Robert W Tipping, Angela Aggrey, Katherine Young, Nicholas A Kartsonis, Joan R Butterton, Amanda Paschke, RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenemnonsusceptible Bacterial Infections, Clinical Infectious Diseases, , ciz 530, https: //doi. org/10. 1093/cid/ciz 530
THE ROLE OF IMIPENEM, CILASTATIN, AND RELEBACTAM CORINA MONTGOMERY, PHARM. D. PGY 1 RESIDENT ERIE VA MEDICAL CENTER
- Slides: 22