The road trip through mole land so far






- Slides: 14

The road trip through mole land so far… • Basic mole-mass-count conversions (Bermuda triangle: divide up, multiply down) • Mole ratio conversion within a compound (`body parts’ relationships)

The mole road ahead. . . • % composition problems and combustion analysis (pp. 94 -103) • Reaction balancing (pp. 105 -108) • Reaction stoichiometry predictions, limiting yields and % yields ( pp. 108 -123)

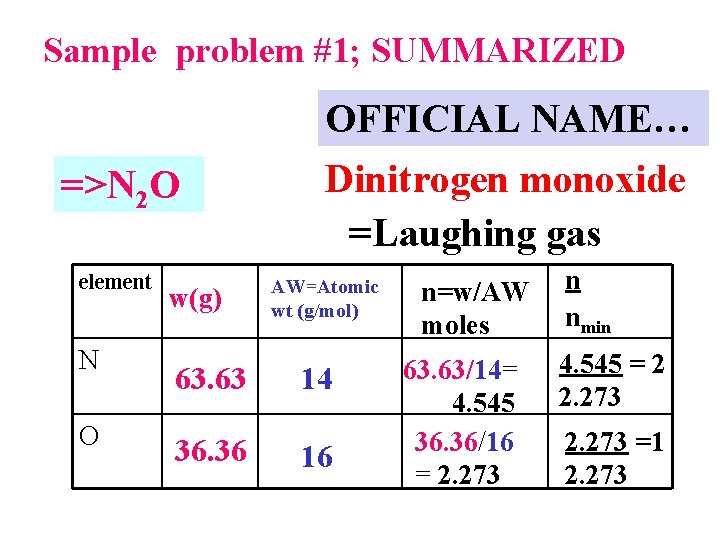
% composition problems: using mole concept to convert weights to formulas Sample % composition problem #1 A compound of N and O contains 63. 63 wt % N and 36. 36 wt % O. What is the empiric formula of the compound ?

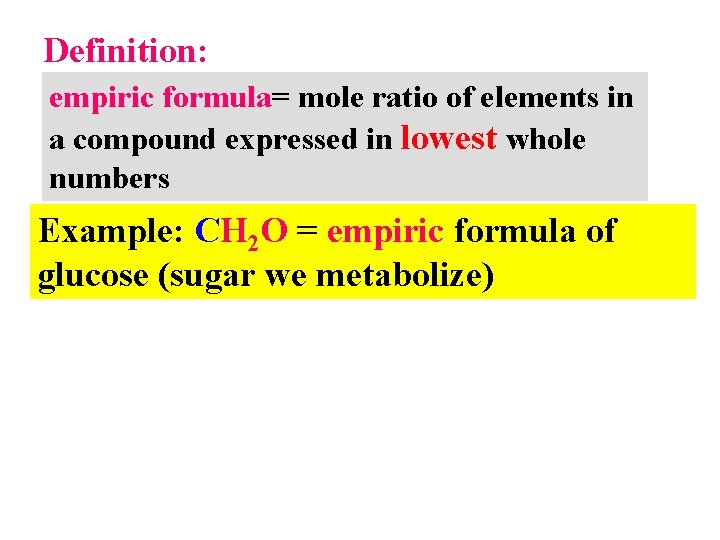
Definition: empiric formula= mole ratio of elements in a compound expressed in lowest whole numbers Example: CH 2 O = empiric formula of glucose (sugar we metabolize)

Empiric formulas (continued) CH 2 O = empiric formula of glucose (sugar we metabolize) Actual molecular formula (what it really has in atom count): C 6 H 12 O 6= (CH 2 O)6 Sugar =Carbo hydrate

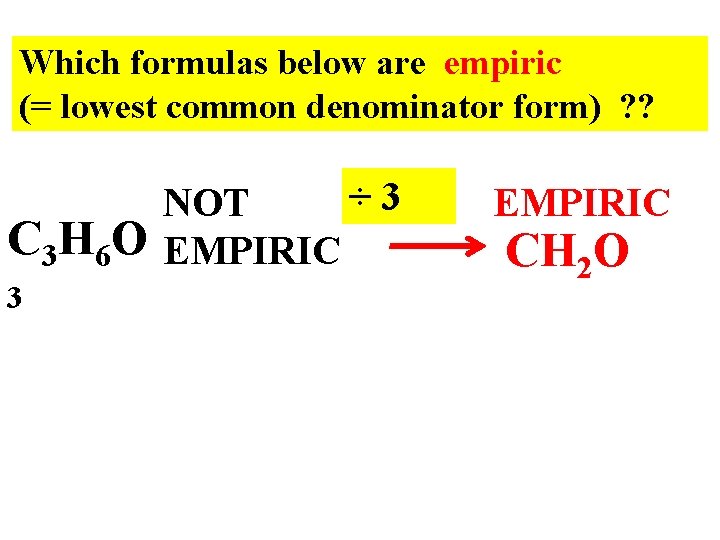
Which formulas below are empiric (= lowest common denominator form) ? ? C 3 H 6 O 3 ÷ 3 NOT EMPIRIC CH 2 O

Which formulas below are empiric (continued) N 3 F 7 ? ? EMPIRIC… 3 & 7 have no shared factors

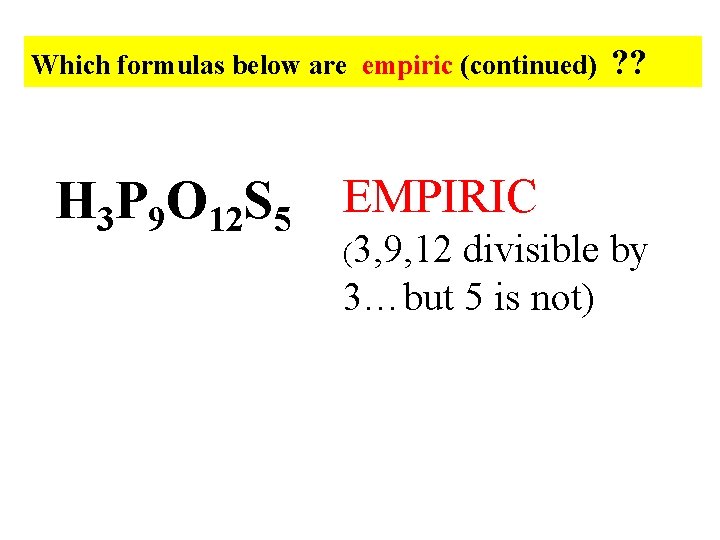
Which formulas below are empiric (continued) H 3 P 9 O 12 S 5 EMPIRIC (3, 9, 12 ? ? divisible by 3…but 5 is not)

% composition Problem 1: empiric formula determination A compound of N and O contains 63. 63% N and 36. 36 %O. What is the empiric formula of the compound? let’s do it on the board…

Sample problem #1; SUMMARIZED OFFICIAL NAME… =>N 2 O element N O w(g) 63. 63 36. 36 Dinitrogen monoxide =Laughing gas AW=Atomic wt (g/mol) 14 16 n=w/AW moles 63. 63/14= 4. 545 36. 36/16 = 2. 273 n nmin 4. 545 = 2 2. 273 =1 2. 273

Joseph Priestley 1772 http: //www. youtube. com/watch? v=_Ha-Zr. UPJ_E

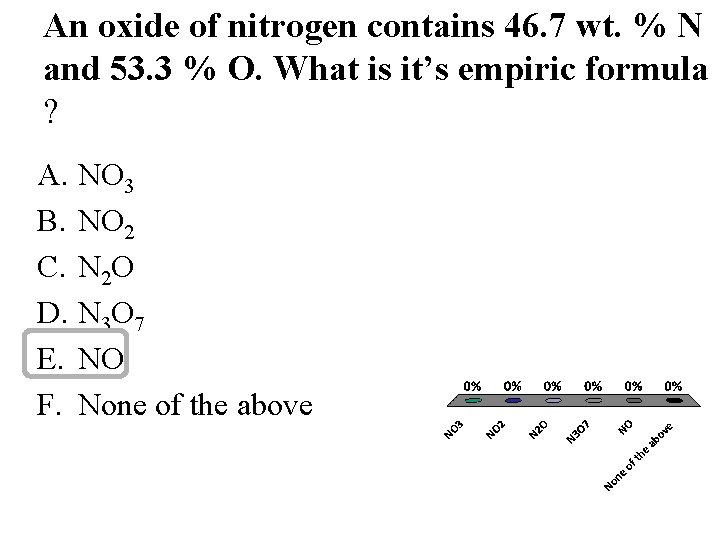
An oxide of nitrogen contains 46. 7 wt. % N and 53. 3 % O. What is it’s empiric formula ? A. NO 3 B. NO 2 C. N 2 O D. N 3 O 7 E. NO F. None of the above

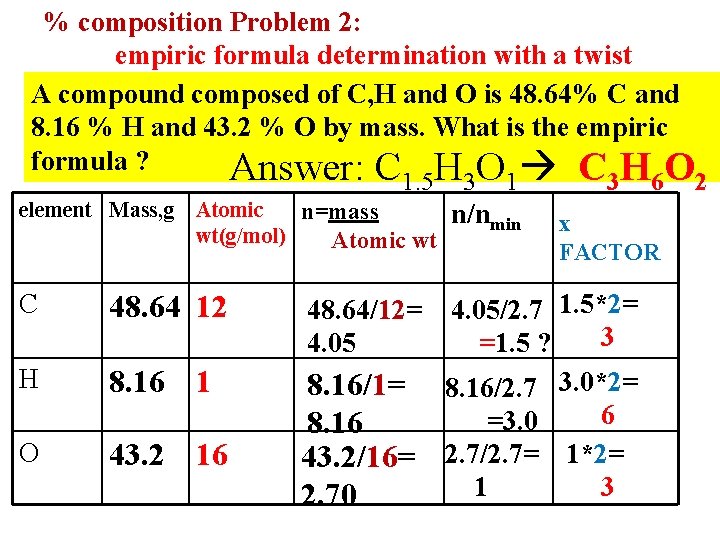
% composition Problem 2: empiric formula determination with a twist A compound composed of C, H and O is 48. 64% C and 8. 16 % H and 43. 2 % O by mass. What is the empiric formula ? Answer: C H O 1. 5 3 1 3 element Mass, g Atomic n=mass wt(g/mol) Atomic wt n/nmin C 48. 64 12 48. 64/12= 4. 05 H 8. 16 1 O 43. 2 16 8. 16/1= 8. 16 43. 2/16= 2. 70 4. 05/2. 7 1. 5*2= 3 =1. 5 ? 8. 16/2. 7 3. 0*2= 6 =3. 0 2. 7/2. 7= 1*2= 1 3 6 x FACTOR 2

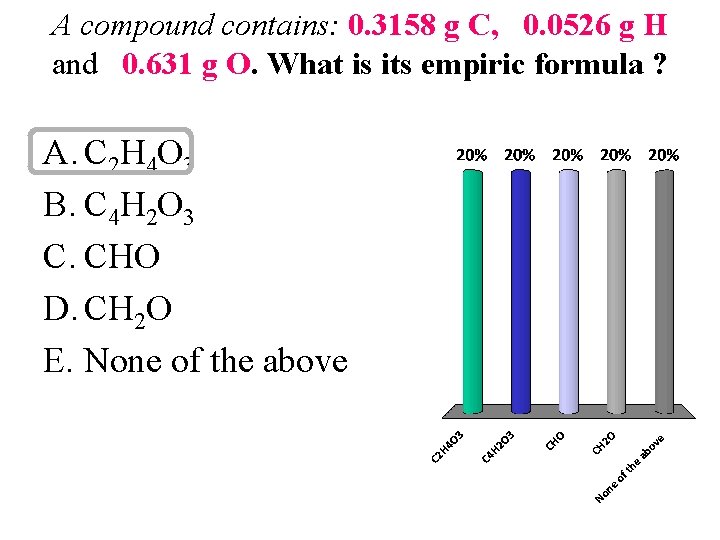
A compound contains: 0. 3158 g C, 0. 0526 g H and 0. 631 g O. What is its empiric formula ? A. C 2 H 4 O 3 B. C 4 H 2 O 3 C. CHO D. CH 2 O E. None of the above