The Repositionable Lotus Aortic Valve Replacement System SixMonth

The Repositionable Lotus Aortic Valve Replacement System: Six-Month Outcomes in the REPRISE I Feasibility Study Ian T. Meredith AM MBBS, Ph. D, FRACP, FCSANZ, FACC On behalf of the REPRISE I Investigators Stephen G. Worthley, MD; Robert J. Whitbourn, MBBS, BMed. Sc, BSc; Paul Antonis, MBBS; Joseph Montarello, MBBS; Andrew E. Newcomb, MBBS; Dominic J. Allocco, MD; Keith D. Dawkins, MD

Disclosures • Consultant Fee / Honoraria / Speaker’s Bureau: – Boston Scientific (Modest) – Medtronic (Modest) The REPRISE I study is sponsored and funded by Boston Scientific Corporation.
Challenges with Current TAVR First generation devices provide significant clinical benefit, but opportunities for improvement remain • • • Controlled deployment Simple, precise and atraumatic aortic/ventricular repositioning Full atraumatic retrieval if required No or trivial paravalvular leakage Simple preparation and loading of valve Decrease complication rate Paravalvular Regurgitation Kodali. NEJM 2012; 366: 1686 Cerebrovascular Accident Stortecky. Euro. Intervention 2012; 8: 623 Vascular Complications Lange. Eur J Cardio-Thoracic Surg 2011; 40: 1105
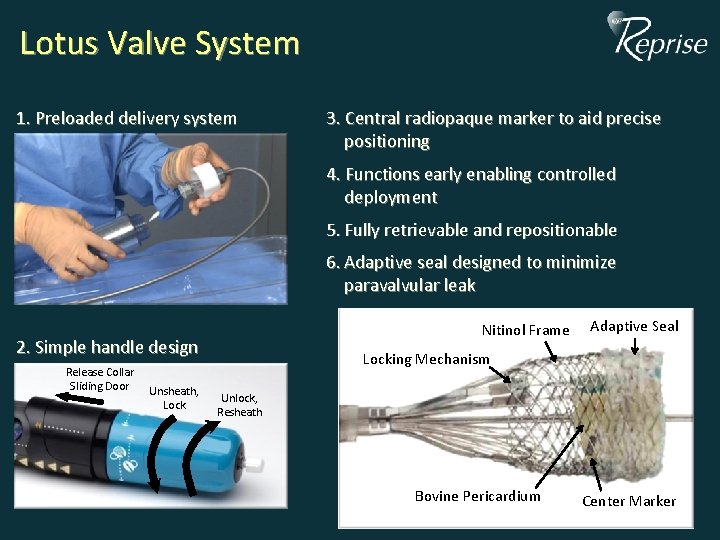
Lotus Valve System 1. Preloaded delivery system 3. Central radiopaque marker to aid precise positioning 4. Functions early enabling controlled deployment 5. Fully retrievable and repositionable 6. Adaptive seal designed to minimize paravalvular leak Nitinol Frame 2. Simple handle design Release Collar Sliding Door Unsheath, Lock Adaptive Seal Locking Mechanism Unlock, Resheath Bovine Pericardium Center Marker
REPRISE I Study Design • OBJECTIVE: Assess acute safety & performance of the Lotus Valve System (23 mm) for TAVR in symptomatic high surgical risk patients with calcified stenotic aortic valves • DESIGN: Prospective, single-arm feasibility study • PRIMARY ENDPOINT: Device success (VARC-1 definition) without in-hospital MACCE through discharge/7 days • SECONDARY ENDPOINTS: – Successful repositioning/retrieval of Lotus Valve System, if attempted – Incidence of prosthetic aortic valve regurgitation by discharge TTE ”Discharge” is defined as discharge or 7 days post-procedure, whichever comes first MACCE: All-cause mortality, peri-procedural MI ≤ 72 hours after index procedure, major stroke, urgent or emergent conversion to surgery or repeat procedure. VARC Device Success: Leon, et al. J Am Coll Cardiol 2011, 57: 253. TTE=transthoracic echocardiography

REPRISE I Key Inclusion Criteria • Age ≥ 70 years • Documented calcified native aortic stenosis – AVA <1. 0 cm² (or AVA index <0. 6 cm²/m²) plus either MPG >40 mm. Hg or jet velocity >4 m/s (by echocardiography) • Symptomatic aortic valve stenosis with NYHA Class ≥II • High risk for surgical AVR – STS score ≥ 8% or euro. SCORE ≥ 20% or documented heart team agreement of high risk due to frailty or comorbidities • Aortic annulus size 19 -22 mm – 23 mm valve size used in study AVA=aortic valve area; MPG=mean pressure gradient; NYHA=New York Heart Association; STS=Society of Thoracic Surgeons
REPRISE I Study Organization Clinical Sites Clinical Events Committee Echocardiography Core Laboratory Sergio Waxman, MD (Interventional Cardiologist, Chair) Victor Davila-Román, MD CVR Consulting, St. Louis, MO, USA Carey Kimmelstiel, MD (Interventional Cardiologist) Gregory Smaroff, MD (Cardiothoracic Surgeon) Roberto Rodriguez, MD (Cardiothoracic Surgeon) Viken Babikian, MD (Neurologist) Electrocardiography Core Laboratory Peter J. Zimetbaum, MD Harvard Clinical Research Institute, Boston, MA, USA
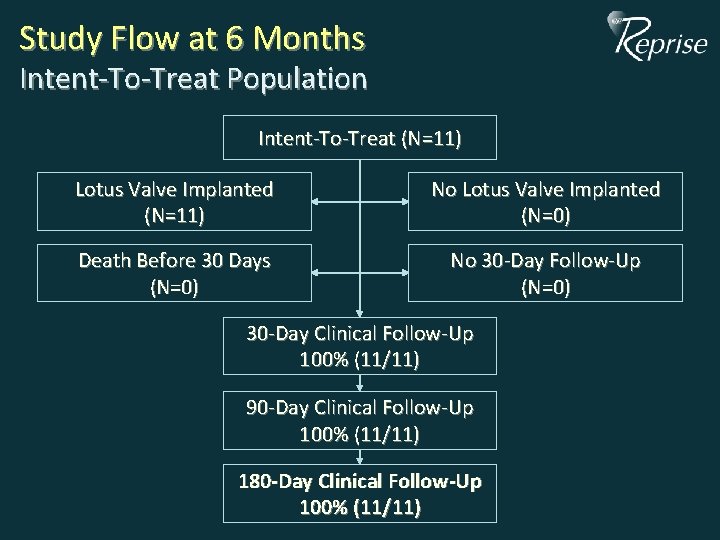
Study Flow at 6 Months Intent-To-Treat Population Intent-To-Treat (N=11) Lotus Valve Implanted (N=11) No Lotus Valve Implanted (N=0) Death Before 30 Days (N=0) No 30 -Day Follow-Up (N=0) 30 -Day Clinical Follow-Up 100% (11/11) 90 -Day Clinical Follow-Up 100% (11/11) 180 -Day Clinical Follow-Up 100% (11/11)
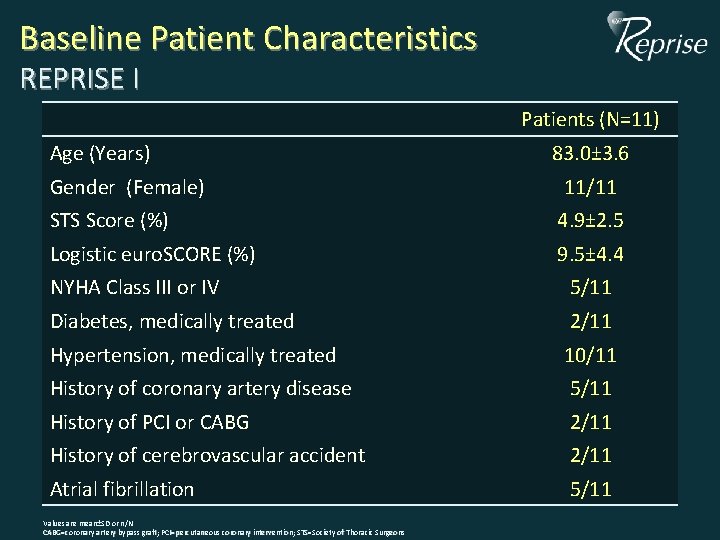
Baseline Patient Characteristics REPRISE I Patients (N=11) Age (Years) Gender (Female) 83. 0± 3. 6 11/11 STS Score (%) 4. 9± 2. 5 Logistic euro. SCORE (%) 9. 5± 4. 4 NYHA Class III or IV 5/11 Diabetes, medically treated 2/11 Hypertension, medically treated 10/11 History of coronary artery disease 5/11 History of PCI or CABG 2/11 History of cerebrovascular accident 2/11 Atrial fibrillation 5/11 Values are mean±SD or n/N CABG=coronary artery bypass graft; PCI=percutaneous coronary intervention; STS=Society of Thoracic Surgeons
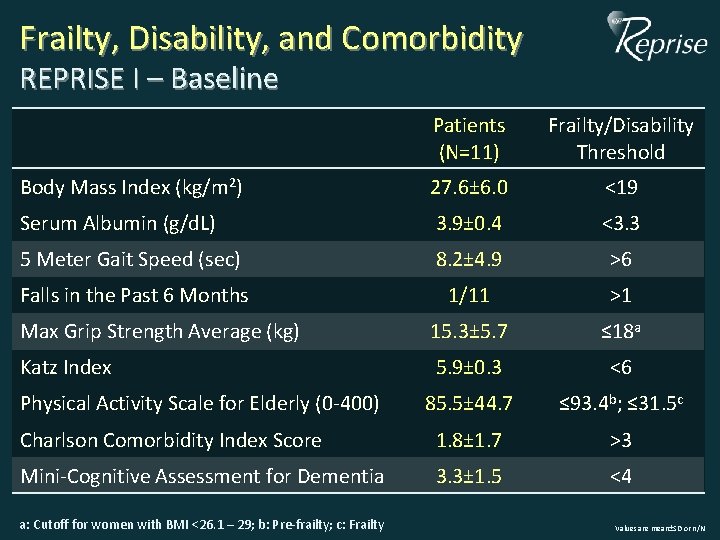
Frailty, Disability, and Comorbidity REPRISE I – Baseline Patients (N=11) Frailty/Disability Threshold Body Mass Index (kg/m 2) 27. 6± 6. 0 <19 Serum Albumin (g/d. L) 3. 9± 0. 4 <3. 3 5 Meter Gait Speed (sec) 8. 2± 4. 9 >6 Falls in the Past 6 Months 1/11 >1 Max Grip Strength Average (kg) 15. 3± 5. 7 ≤ 18 a Katz Index 5. 9± 0. 3 <6 85. 5± 44. 7 ≤ 93. 4 b; ≤ 31. 5 c Charlson Comorbidity Index Score 1. 8± 1. 7 >3 Mini-Cognitive Assessment for Dementia 3. 3± 1. 5 <4 Physical Activity Scale for Elderly (0 -400) a: Cutoff for women with BMI <26. 1 – 29; b: Pre-frailty; c: Frailty Values are mean±SD or n/N
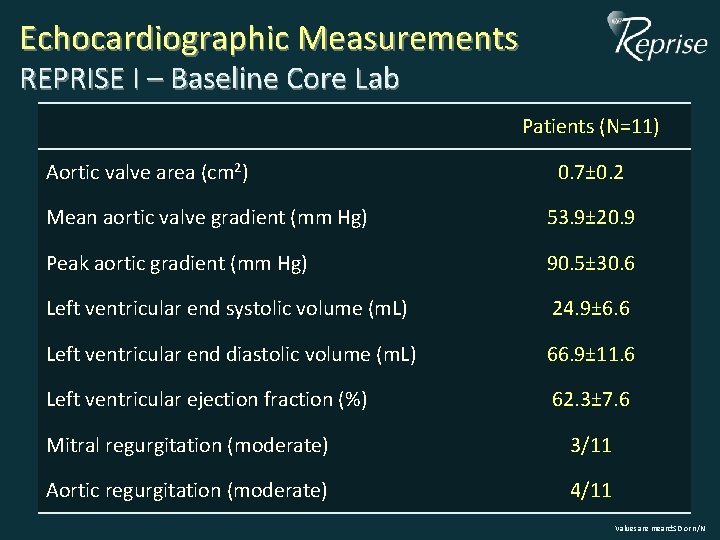
Echocardiographic Measurements REPRISE I – Baseline Core Lab Patients (N=11) Aortic valve area (cm 2) 0. 7± 0. 2 Mean aortic valve gradient (mm Hg) 53. 9± 20. 9 Peak aortic gradient (mm Hg) 90. 5± 30. 6 Left ventricular end systolic volume (m. L) 24. 9± 6. 6 Left ventricular end diastolic volume (m. L) 66. 9± 11. 6 Left ventricular ejection fraction (%) 62. 3± 7. 6 Mitral regurgitation (moderate) 3/11 Aortic regurgitation (moderate) 4/11 Values are mean±SD or n/N
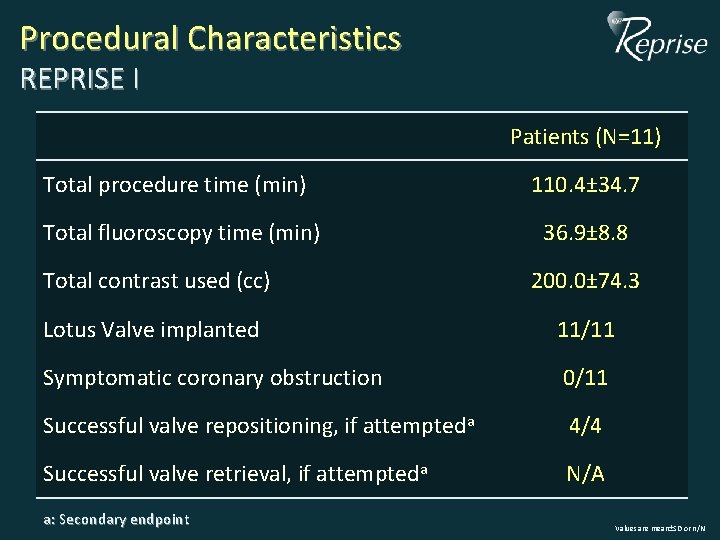
Procedural Characteristics REPRISE I Patients (N=11) Total procedure time (min) 110. 4± 34. 7 Total fluoroscopy time (min) 36. 9± 8. 8 Total contrast used (cc) 200. 0± 74. 3 Lotus Valve implanted 11/11 Symptomatic coronary obstruction 0/11 Successful valve repositioning, if attempteda 4/4 Successful valve retrieval, if attempteda N/A a: Secondary endpoint Values are mean±SD or n/N
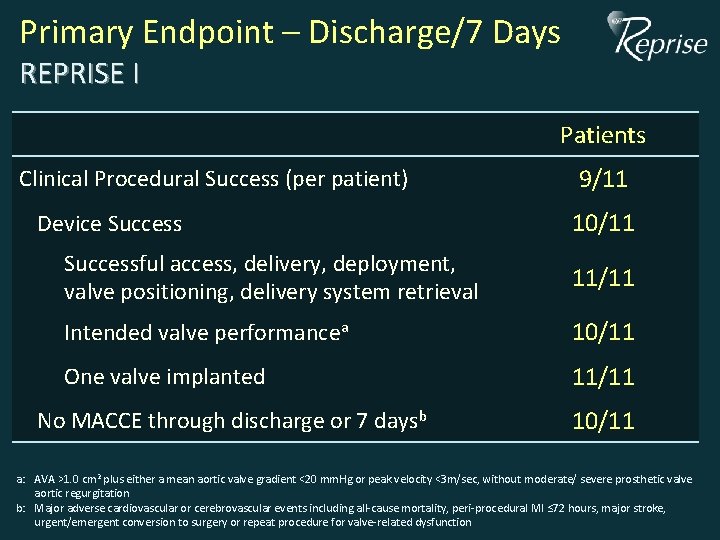
Primary Endpoint – Discharge/7 Days REPRISE I Patients Clinical Procedural Success (per patient) Device Success 9/11 10/11 Successful access, delivery, deployment, valve positioning, delivery system retrieval 11/11 Intended valve performancea 10/11 One valve implanted 11/11 No MACCE through discharge or 7 daysb 10/11 a: AVA >1. 0 cm 2 plus either a mean aortic valve gradient <20 mm. Hg or peak velocity <3 m/sec, without moderate/ severe prosthetic valve aortic regurgitation b: Major adverse cardiovascular or cerebrovascular events including all-cause mortality, peri-procedural MI ≤ 72 hours, major stroke, urgent/emergent conversion to surgery or repeat procedure for valve-related dysfunction
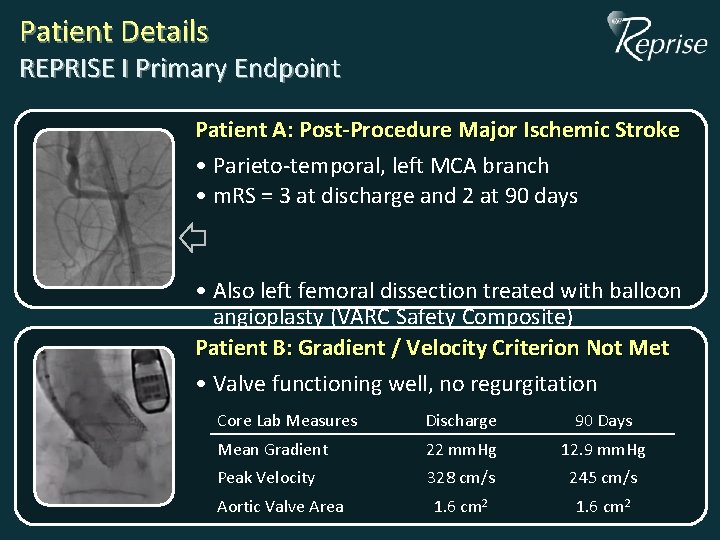
Patient Details REPRISE I Primary Endpoint Patient A: Post-Procedure Major Ischemic Stroke • Parieto-temporal, left MCA branch • m. RS = 3 at discharge and 2 at 90 days • Also left femoral dissection treated with balloon angioplasty (VARC Safety Composite) Patient B: Gradient / Velocity Criterion Not Met • Valve functioning well, no regurgitation Core Lab Measures Discharge 90 Days Mean Gradient 22 mm. Hg 12. 9 mm. Hg Peak Velocity 328 cm/s 245 cm/s 1. 6 cm 2 Aortic Valve Area
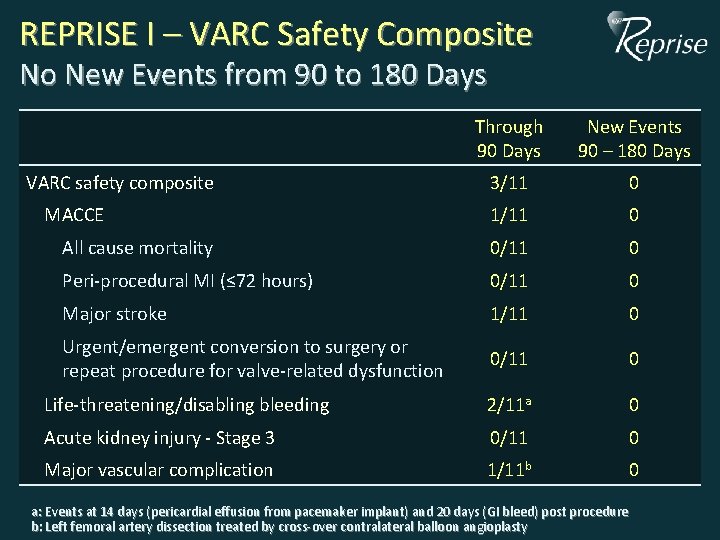
REPRISE I – VARC Safety Composite No New Events from 90 to 180 Days Through 30 Days 90 Days New Events 90 – 180 Days 3/11 0 1/11 0 All cause mortality 0/11 0 Peri-procedural MI (≤ 72 hours) 0/11 0 Major stroke 1/11 0 Urgent/emergent conversion to surgery or repeat procedure for valve-related dysfunction 0/11 0 Life-threatening/disabling bleeding 2/11 a 0 Acute kidney injury - Stage 3 0/11 0 Major vascular complication 1/11 b 0 VARC safety composite MACCE a: Events at 14 days (pericardial effusion from pacemaker implant) and 20 days (GI bleed) post procedure b: Left femoral artery dissection treated by cross-over contralateral balloon angioplasty
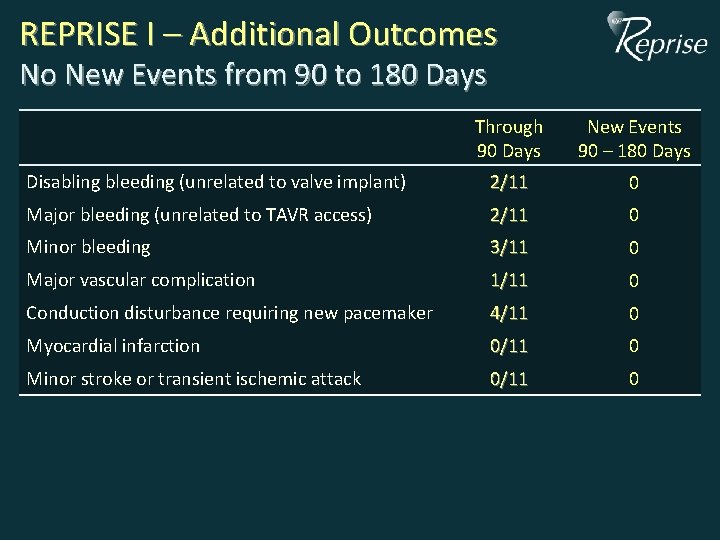
REPRISE I – Additional Outcomes No New Events from 90 to 180 Days Through 90 Days New Events 90 – 180 Days Disabling bleeding (unrelated to valve implant) 2/11 0 Major bleeding (unrelated to TAVR access) 2/11 0 Minor bleeding 3/11 0 Major vascular complication 1/11 0 Conduction disturbance requiring new pacemaker 4/11 0 Myocardial infarction 0/11 0 Minor stroke or transient ischemic attack 0/11 0
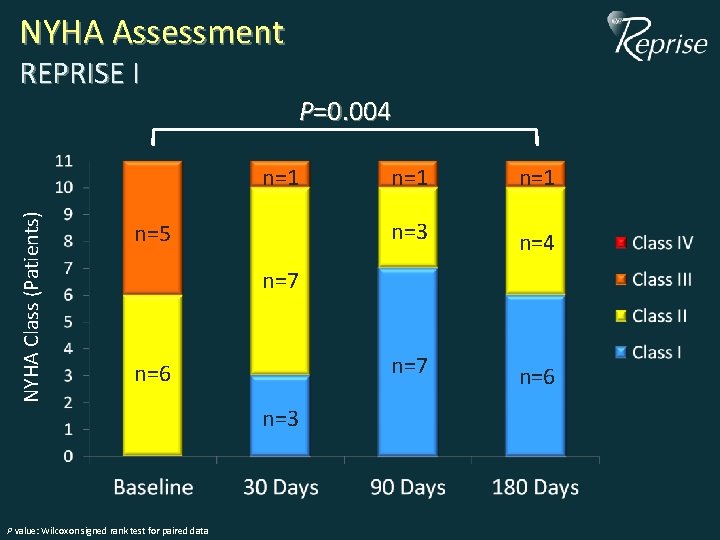
NYHA Assessment REPRISE I P=0. 004 NYHA Class (Patients) n=1 n=5 n=1 n=3 n=4 n=7 n=6 n=3 P value: Wilcoxon signed rank test for paired data
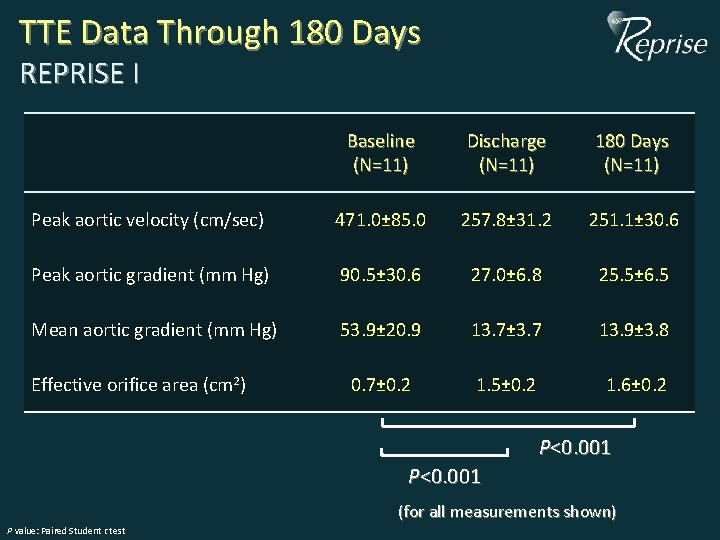
TTE Data Through 180 Days REPRISE I Baseline (N=11) Discharge (N=11) 180 Days (N=11) Peak aortic velocity (cm/sec) 471. 0± 85. 0 257. 8± 31. 2 251. 1± 30. 6 Peak aortic gradient (mm Hg) 90. 5± 30. 6 27. 0± 6. 8 25. 5± 6. 5 Mean aortic gradient (mm Hg) 53. 9± 20. 9 13. 7± 3. 7 13. 9± 3. 8 0. 7± 0. 2 1. 5± 0. 2 1. 6± 0. 2 Effective orifice area (cm 2) P<0. 001 (for all measurements shown) P value: Paired Student t test
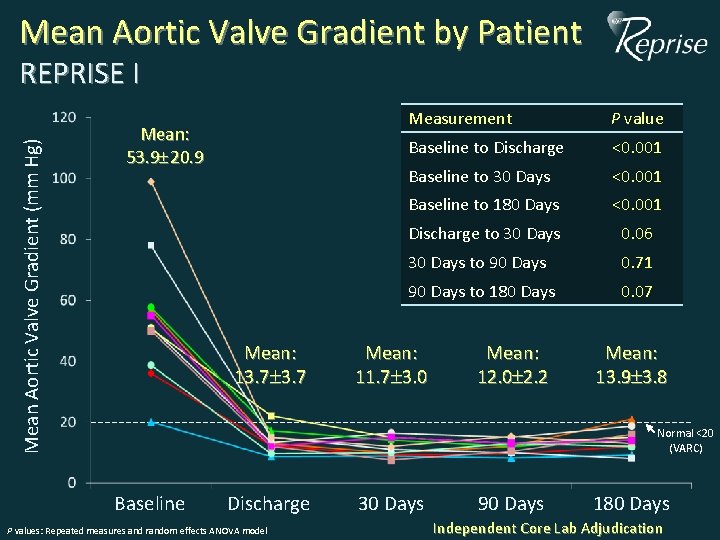
Mean Aortic Valve Gradient by Patient Mean Aortic Valve Gradient (mm Hg) REPRISE I Mean: 53. 9 20. 9 Mean: 13. 7 Measurement P value Baseline to Discharge <0. 001 Baseline to 30 Days <0. 001 Baseline to 180 Days <0. 001 Discharge to 30 Days 0. 06 30 Days to 90 Days 0. 71 90 Days to 180 Days 0. 07 Mean: 11. 7 3. 0 Mean: 12. 0 2. 2 Mean: 13. 9 3. 8 Normal <20 (VARC) Baseline Discharge P values: Repeated measures and random effects ANOVA model 30 Days 90 Days 180 Days Independent Core Lab Adjudication
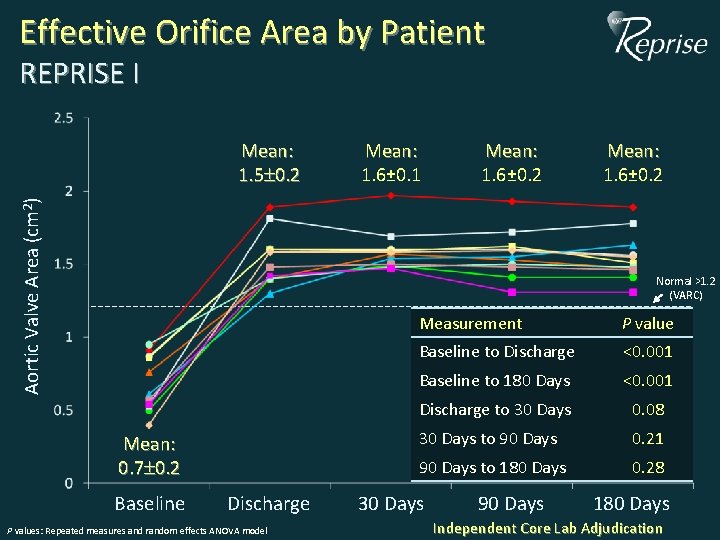
Effective Orifice Area by Patient REPRISE I Aortic Valve Area (cm 2) Mean: 1. 5 0. 2 Mean: 1. 6± 0. 1 Mean: 1. 6± 0. 2 Normal >1. 2 (VARC) Mean: 0. 7 0. 2 Baseline Discharge P values: Repeated measures and random effects ANOVA model Measurement P value Baseline to Discharge <0. 001 Baseline to 180 Days <0. 001 Discharge to 30 Days 0. 08 30 Days to 90 Days 0. 21 90 Days to 180 Days 0. 28 30 Days 90 Days 180 Days Independent Core Lab Adjudication
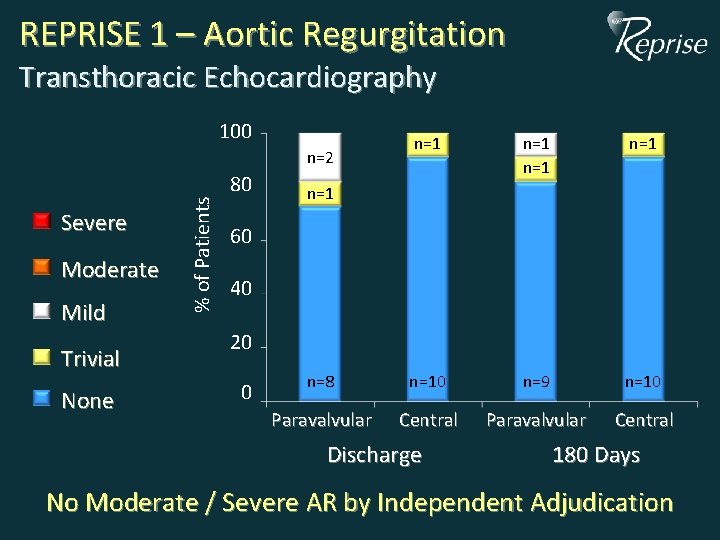
REPRISE 1 – Aortic Regurgitation Transthoracic Echocardiography 100 n=1 n=1 n=8 n=10 n=9 n=10 Paravalvular Central Severe Moderate Mild Trivial None % of Patients n=2 80 n=1 60 40 20 0 Discharge 180 Days No Moderate / Severe AR by Independent Adjudication
REPRISE I Summary of 180 -Day Data • MACCE: 1/11 – Major stroke, post-procedure • VARC 180 -day safety composite: 3/11 – 2 disabling bleeds (not related to valve implant) (<30 days) – 1 major vascular complication (femoral dissection) (Day 0) • No deaths or myocardial infarctions • Significant improvements in NYHA class and TTE measures • No moderate or severe aortic regurgitation – 10/11 patients with no or trivial AR, 1/11 with mild AR No new events from 90 to 180 days

REPRISE I 180 -Day Conclusions • Lotus Valve can be positioned precisely & successfully with minimal aortic regurgitation after placement • The safety & efficacy of the Lotus Valve are sustained out to 6 months • The ongoing REPRISE II Study will confirm these data in a larger patient population.
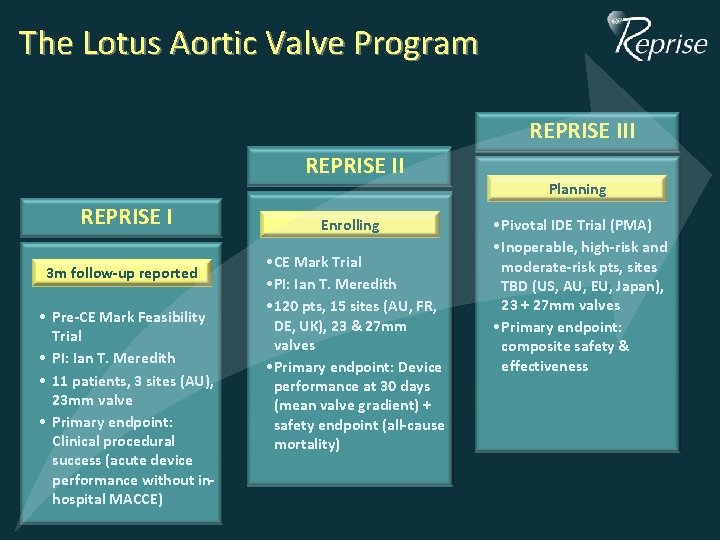
The Lotus Aortic Valve Program REPRISE III REPRISE I 3 m follow-up reported • Pre-CE Mark Feasibility Trial • PI: Ian T. Meredith • 11 patients, 3 sites (AU), 23 mm valve • Primary endpoint: Clinical procedural success (acute device performance without inhospital MACCE) Enrolling CE Mark Trial • • CE PI: Ian T. T. Meredith • • PI: 120 pts, 15 15 sites (AU, FR, • • 120 DE, UK), 23 + 27 mm DE, UK), 23 & 27 mm valves • Primary endpoint: Device • Primary endpoint: performance at 30 Device days performance at 30 days (mean valve gradient) + (mean gradient) + safety valve endpoint (all-cause mortality) safety endpoint (all-cause mortality) Planning • Pivotal IDE Trial (PMA) • Inoperable, high-risk and moderate-risk pts, sites TBD (US, AU, EU, Japan), 23 + 27 mm valves • Primary endpoint: composite safety & effectiveness

Thank You!
- Slides: 25