The Radiatively Important Trace Species RITS Data Recovery
- Slides: 1

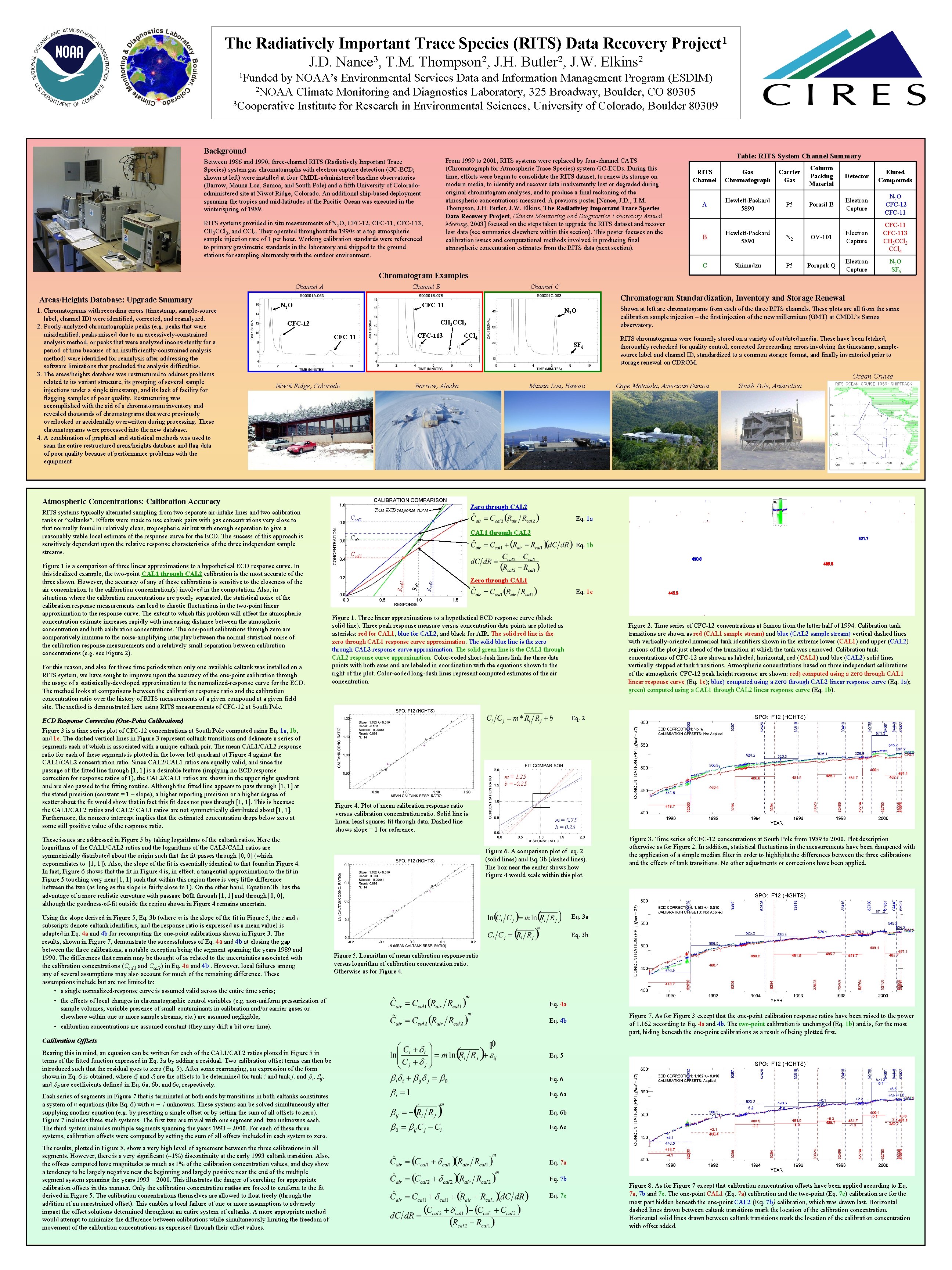
The Radiatively Important Trace Species (RITS) Data Recovery 1 Project J. D. Nance 3, T. M. Thompson 2, J. H. Butler 2, J. W. Elkins 2 1 Funded by NOAA’s Environmental Services Data and Information Management Program (ESDIM) 2 NOAA Climate Monitoring and Diagnostics Laboratory, 325 Broadway, Boulder, CO 80305 3 Cooperative Institute for Research in Environmental Sciences, University of Colorado, Boulder 80309 Background Table: RITS System Channel Summary From 1999 to 2001, RITS systems were replaced by four-channel CATS (Chromatograph for Atmospheric Trace Species) system GC-ECDs. During this time, efforts were begun to consolidate the RITS dataset, to renew its storage on modern media, to identify and recover data inadvertently lost or degraded during original chromatogram analyses, and to produce a final reckoning of the atmospheric concentrations measured. A previous poster [Nance, J. D. , T. M. Thompson, J. H. Butler, J. W. Elkins, The Radiativley Important Trace Species Data Recovery Project, Climate Monitoring and Diagnostics Laboratory Annual Meeting, 2003] focused on the steps taken to upgrade the RITS dataset and recover lost data (see summaries elsewhere within this section). This poster focuses on the calibration issues and computational methods involved in producing final atmospheric concentration estimates from the RITS data (next section). Between 1986 and 1990, three-channel RITS (Radiatively Important Trace Species) system gas chromatographs with electron capture detection (GC-ECD; shown at left) were installed at four CMDL-administered baseline observatories (Barrow, Mauna Loa, Samoa, and South Pole) and a fifth University of Coloradoadministered site at Niwot Ridge, Colorado. An additional ship-based deployment spanning the tropics and mid-latitudes of the Pacific Ocean was executed in the winter/spring of 1989. RITS systems provided in situ measurements of N 2 O, CFC-12, CFC-113, CH 3 CCl 3, and CCl 4. They operated throughout the 1990 s at a top atmospheric sample injection rate of 1 per hour. Working calibration standards were referenced to primary gravimetric standards in the laboratory and shipped to the ground stations for sampling alternately with the outdoor environment. RITS Channel Gas Chromatograph A Hewlett-Packard 5890 Carrier Gas P 5 Column Packing Material Detector Eluted Compounds Porasil B Electron Capture N 2 O CFC-12 CFC-11 B Hewlett-Packard 5890 N 2 OV-101 Electron Capture CFC-113 CH 3 CCl 3 CCl 4 C Shimadzu P 5 Porapak Q Electron Capture N 2 O SF 6 Chromatogram Examples Channel A Areas/Heights Database: Upgrade Summary 1. Chromatograms with recording errors (timestamp, sample-source label, channel ID) were identified, corrected, and reanalyzed. 2. Poorly-analyzed chromatographic peaks (e. g. peaks that were misidentified, peaks missed due to an excessively-constrained analysis method, or peaks that were analyzed inconsistently for a period of time because of an insufficiently-constrained analysis method) were identified for reanalysis after addressing the software limitations that precluded the analysis difficulties. 3. The areas/heights database was restructured to address problems related to its variant structure, its grouping of several sample injections under a single timestamp, and its lack of facility for flagging samples of poor quality. Restructuring was accomplished with the aid of a chromatogram inventory and revealed thousands of chromatograms that were previously overlooked or accidentally overwritten during processing. These chromatograms were processed into the new database. 4. A combination of graphical and statistical methods was used to scan the entire restructured areas/heights database and flag data of poor quality because of performance problems with the equipment Channel B Channel C Chromatogram Standardization, Inventory and Storage Renewal CFC-11 N 2 O N 22 O O N CH 3 CCl 3 CFC-12 CFC-113 CFC-11 CCl 4 SF 6 Shown at left are chromatograms from each of the three RITS channels. These plots are all from the same calibration sample injection – the first injection of the new millennium (GMT) at CMDL’s Samoa observatory. RITS chromatograms were formerly stored on a variety of outdated media. These have been fetched, thoroughly rechecked for quality control, corrected for recording errors involving the timestamp, samplesource label and channel ID, standardized to a common storage format, and finally inventoried prior to storage renewal on CDROM. Ocean Cruise Niwot Ridge, Colorado Barrow, Alaska Atmospheric Concentrations: Calibration Accuracy For this reason, and also for those time periods when only one available caltank was installed on a RITS system, we have sought to improve upon the accuracy of the one-point calibration through the usage of a statistically-developed approximation to the normalized-response curve for the ECD. The method looks at comparisons between the calibration response ratio and the calibration concentration ratio over the history of RITS measurements of a given compound at a given field site. The method is demonstrated here using RITS measurements of CFC-12 at South Pole. CAL 1 through CAL 2 Cair 521. 7 Eq. 1 b Ccal 1 490. 8 Rcal 2 489. 8 Zero through CAL 1 Eq. 1 c Figure 1. Three linear approximations to a hypothetical ECD response curve (black solid line). Three peak response measure versus concentration data points are plotted as asterisks: red for CAL 1, blue for CAL 2, and black for AIR. The solid red line is the zero through CAL 1 response curve approximation. The solid blue line is the zero through CAL 2 response curve approximation. The solid green line is the CAL 1 through CAL 2 response curve approximation. Color-coded short-dash lines link the three data points with both axes and are labeled in coordination with the equations shown to the right of the plot. Color-coded long-dash lines represent computed estimates of the air concentration. • the effects of local changes in chromatographic control variables (e. g. non-uniform pressurization of sample volumes, variable presence of small contaminants in calibration and/or carrier gases or elsewhere within one or more sample streams, etc. ) are assumed negligible; • calibration concentrations are assumed constant (they may drift a bit over time). 445. 5 Figure 2. Time series of CFC-12 concentrations at Samoa from the latter half of 1994. Calibration tank transitions are shown as red (CAL 1 sample stream) and blue (CAL 2 sample stream) vertical dashed lines with vertically-oriented numerical tank identifiers shown in the extreme lower (CAL 1) and upper (CAL 2) regions of the plot just ahead of the transition at which the tank was removed. Calibration tank concentrations of CFC-12 are shown as labeled, horizontal, red (CAL 1) and blue (CAL 2) solid lines vertically stepped at tank transitions. Atmospheric concentrations based on three independent calibrations of the atmospheric CFC-12 peak height response are shown: red) computed using a zero through CAL 1 linear response curve (Eq. 1 c); blue) computed using a zero through CAL 2 linear response curve (Eq. 1 a); green) computed using a CAL 1 through CAL 2 linear response curve (Eq. 1 b). Eq. 2 m = 1. 25 b = -0. 25 Figure 4. Plot of mean calibration response ratio versus calibration concentration ratio. Solid line is linear least squares fit through data. Dashed line shows slope = 1 for reference. These issues are addressed in Figure 5 by taking logarithms of the caltank ratios. Here the logarithms of the CAL 1/CAL 2 ratios and the logarithms of the CAL 2/CAL 1 ratios are symmetrically distributed about the origin such that the fit passes through [0, 0] (which exponentiates to [1, 1]). Also, the slope of the fit is essentially identical to that found in Figure 4. In fact, Figure 6 shows that the fit in Figure 4 is, in effect, a tangential approximation to the fit in Figure 5 touching very near [1, 1] such that within this region there is very little difference between the two (as long as the slope is fairly close to 1). On the other hand, Equation 3 b has the advantage of a more realistic curvature with passage both through [1, 1] and through [0, 0], although the goodness-of-fit outside the region shown in Figure 4 remains uncertain. Using the slope derived in Figure 5, Eq. 3 b (where m is the slope of the fit in Figure 5, the i and j subscripts denote caltank identifiers, and the response ratio is expressed as a mean value) is adapted in Eq. 4 a and 4 b for recomputing the one-point calibrations shown in Figure 3. The results, shown in Figure 7, demonstrate the successfulness of Eq. 4 a and 4 b at closing the gap between the three calibrations, a notable exception being the segment spanning the years 1989 and 1990. The differences that remain may be thought of as related to the uncertainties associated with the calibration concentrations (Ccal 1 and Ccal 2) in Eq. 4 a and 4 b. However, local failures among any of several assumptions may also account for much of the remaining difference. These assumptions include but are not limited to: • a single normalized-response curve is assumed valid across the entire time series; South Pole, Antarctica Eq. 1 a ECD Response Correction (One-Point Calibrations) Figure 3 is a time series plot of CFC-12 concentrations at South Pole computed using Eq. 1 a, 1 b, and 1 c. The dashed vertical lines in Figure 3 represent caltank transitions and delineate a series of segments each of which is associated with a unique caltank pair. The mean CAL 1/CAL 2 response ratio for each of these segments is plotted in the lower left quadrant of Figure 4 against the CAL 1/CAL 2 concentration ratio. Since CAL 2/CAL 1 ratios are equally valid, and since the passage of the fitted line through [1, 1] is a desirable feature (implying no ECD response correction for response ratios of 1), the CAL 2/CAL 1 ratios are shown in the upper right quadrant and are also passed to the fitting routine. Although the fitted line appears to pass through [1, 1] at the stated precision (constant = 1 – slope), a higher reporting precision or a higher degree of scatter about the fit would show that in fact this fit does not pass through [1, 1]. This is because the CAL 1/CAL 2 ratios and CAL 2/ CAL 1 ratios are not symmetrically distributed about [1, 1]. Furthermore, the nonzero intercept implies that the estimated concentration drops below zero at some still positive value of the response ratio. Cape Matatula, American Samoa Zero through CAL 2 Ccal 2 Rair Figure 1 is a comparison of three linear approximations to a hypothetical ECD response curve. In this idealized example, the two-point CAL 1 through CAL 2 calibration is the most accurate of the three shown. However, the accuracy of any of these calibrations is sensitive to the closeness of the air concentration to the calibration concentration(s) involved in the computation. Also, in situations where the calibration concentrations are poorly separated, the statistical noise of the calibration response measurements can lead to chaotic fluctuations in the two-point linear approximation to the response curve. The extent to which this problem will affect the atmospheric concentration estimate increases rapidly with increasing distance between the atmospheric concentration and both calibration concentrations. The one-point calibrations through zero are comparatively immune to the noise-amplifying interplay between the normal statistical noise of the calibration response measurements and a relatively small separation between calibration concentrations (e. g. see Figure 2). True ECD response curve Rcal 1 RITS systems typically alternated sampling from two separate air-intake lines and two calibration tanks or “caltanks”. Efforts were made to use caltank pairs with gas concentrations very close to that normally found in relatively clean, tropospheric air but with enough separation to give a reasonably stable local estimate of the response curve for the ECD. The success of this approach is sensitively dependent upon the relative response characteristics of the three independent sample streams. Mauna Loa, Hawaii m = 0. 75 b = 0. 25 Figure 6. A comparison plot of eq. 2 (solid lines) and Eq. 3 b (dashed lines). The box near the center shows how Figure 4 would scale within this plot. Figure 3. Time series of CFC-12 concentrations at South Pole from 1989 to 2000. Plot description otherwise as for Figure 2. In addition, statistical fluctuations in the measurements have been dampened with the application of a simple median filter in order to highlight the differences between the three calibrations and the effects of tank transitions. No other adjustments or corrections have been applied. Eq. 3 a Eq. 3 b Figure 5. Logarithm of mean calibration response ratio versus logarithm of calibration concentration ratio. Otherwise as for Figure 4. Eq. 4 a Eq. 4 b Figure 7. As for Figure 3 except that the one-point calibration response ratios have been raised to the power of 1. 162 according to Eq. 4 a and 4 b. The two-point calibration is unchanged (Eq. 1 b) and is, for the most part, hiding beneath the one-point calibrations as a result of being plotted first. Calibration Offsets Bearing this in mind, an equation can be written for each of the CAL 1/CAL 2 ratios plotted in Figure 5 in terms of the fitted function expressed in Eq. 3 a by adding a residual. Two calibration offset terms can then be introduced such that the residual goes to zero (Eq. 5). After some rearranging, an expression of the form shown in Eq. 6 is obtained, where di and dj are the offsets to be determined for tank i and tank j, and bi, bij, and b 0 are coefficients defined in Eq. 6 a, 6 b, and 6 c, respectively. Eq. 5 Each series of segments in Figure 7 that is terminated at both ends by transitions in both caltanks constitutes a system of n equations (like Eq. 6) with n + 1 unknowns. These systems can be solved simultaneously after supplying another equation (e. g. by presetting a single offset or by setting the sum of all offsets to zero). Figure 7 includes three such systems. The first two are trivial with one segment and two unknowns each. The third system includes multiple segments spanning the years 1993 – 2000. For each of these three systems, calibration offsets were computed by setting the sum of all offsets included in each system to zero. Eq. 6 a The results, plotted in Figure 8, show a very high level of agreement between the three calibrations in all segments. However, there is a very significant (~1%) discontinuity at the early 1993 caltank transition. Also, the offsets computed have magnitudes as much as 1% of the calibration concentration values, and they show a tendency to be largely negative near the beginning and largely positive near the end of the multiple segment system spanning the years 1993 – 2000. This illustrates the danger of searching for appropriate calibration offsets in this manner. Only the calibration concentration ratios are forced to conform to the fit derived in Figure 5. The calibration concentrations themselves are allowed to float freely (through the addition of an unrestrained offset). This enables a local failure of one or more assumptions to adversely impact the offset solutions determined throughout an entire system of caltanks. A more appropriate method would attempt to minimize the difference between calibrations while simultaneously limiting the freedom of movement of the calibration concentrations as expressed through their offset values. Eq. 6 b Eq. 6 c Eq. 7 a Eq. 7 b Eq. 7 c Figure 8. As for Figure 7 except that calibration concentration offsets have been applied according to Eq. 7 a, 7 b and 7 c. The one-point CAL 1 (Eq. 7 a) calibration and the two-point (Eq. 7 c) calibration are for the most part hidden beneath the one-point CAL 2 (Eq. 7 b) calibration, which was drawn last. Horizontal dashed lines drawn between caltank transitions mark the location of the calibration concentration. Horizontal solid lines drawn between caltank transitions mark the location of the calibration concentration with offset added.