The Quantum Mechanics of Fine Structure Lines OI
![The Quantum Mechanics of Fine Structure Lines: [OI], [OIII]; [CII], [CI] Hans Zinnecker Deutsches The Quantum Mechanics of Fine Structure Lines: [OI], [OIII]; [CII], [CI] Hans Zinnecker Deutsches](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-1.jpg)
![Outline Motivation: [OI] 1 s^2 2 p^4 S=1 Discovery stories of FSL (Martin Harwit) Outline Motivation: [OI] 1 s^2 2 p^4 S=1 Discovery stories of FSL (Martin Harwit)](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-2.jpg)
![Oxygen [OI] spin-orbit states 3 P states (S=1, L=1) fine structure lines 1 D Oxygen [OI] spin-orbit states 3 P states (S=1, L=1) fine structure lines 1 D](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-5.jpg)
![Oxygen [OI] multi-electron system: outer sub-shell (4 electrons): 2 p^4 Oxygen [OI] multi-electron system: outer sub-shell (4 electrons): 2 p^4](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-6.jpg)
![[OI] fact sheet • • • Ionisation potential: 13. 62 e. V (vs. HI [OI] fact sheet • • • Ionisation potential: 13. 62 e. V (vs. HI](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-7.jpg)
![Fact sheet [CII], [CI], and [OIII] • [CII] - 157. 7 mu, E = Fact sheet [CII], [CI], and [OIII] • [CII] - 157. 7 mu, E =](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-8.jpg)

![Summary q The 63 mu / 145 mu FSL lines of [OI] owe their Summary q The 63 mu / 145 mu FSL lines of [OI] owe their](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-10.jpg)
- Slides: 10
![The Quantum Mechanics of Fine Structure Lines OI OIII CII CI Hans Zinnecker Deutsches The Quantum Mechanics of Fine Structure Lines: [OI], [OIII]; [CII], [CI] Hans Zinnecker Deutsches](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-1.jpg)
The Quantum Mechanics of Fine Structure Lines: [OI], [OIII]; [CII], [CI] Hans Zinnecker Deutsches SOFIA Institut NASA-Ames and Univ. Stuttgart FSL Workshop, 8 -11 June 2015 MPIA Heidelberg
![Outline Motivation OI 1 s2 2 p4 S1 Discovery stories of FSL Martin Harwit Outline Motivation: [OI] 1 s^2 2 p^4 S=1 Discovery stories of FSL (Martin Harwit)](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-2.jpg)
Outline Motivation: [OI] 1 s^2 2 p^4 S=1 Discovery stories of FSL (Martin Harwit) spectro. notation (term symbols) : S, L, J Spin-orbit-coupling (Russell-Saunders 1925) Pauli’s exclusion principle Hund’s rules (ground states) CASE by CASE: - particularly why for [OI] spin S = 1 (triplet) - [OIII] and [CI] similar e-config, [CII] S=1/2 - energy levels, critical densities, ionis. pot.

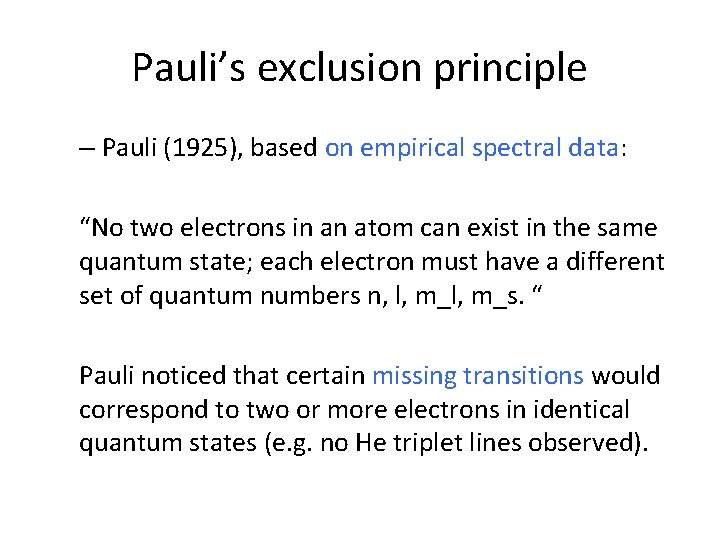
Pauli’s exclusion principle – Pauli (1925), based on empirical spectral data: “No two electrons in an atom can exist in the same quantum state; each electron must have a different set of quantum numbers n, l, m_s. “ Pauli noticed that certain missing transitions would correspond to two or more electrons in identical quantum states (e. g. no He triplet lines observed).

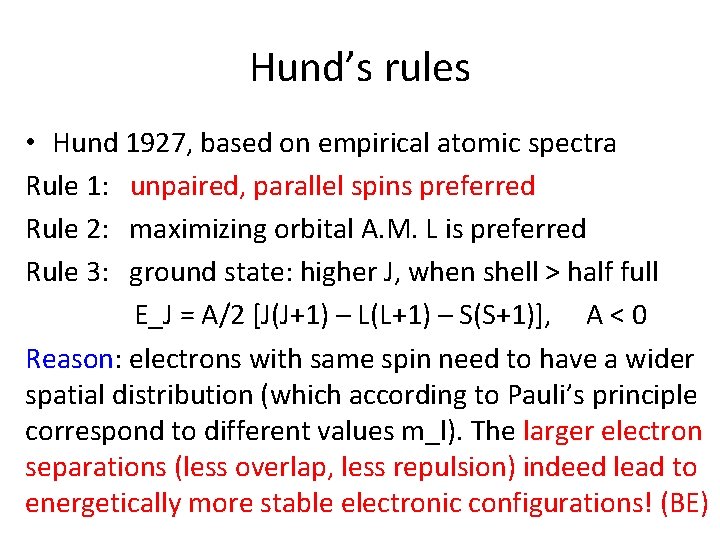
Hund’s rules • Hund 1927, based on empirical atomic spectra Rule 1: unpaired, parallel spins preferred Rule 2: maximizing orbital A. M. L is preferred Rule 3: ground state: higher J, when shell > half full E_J = A/2 [J(J+1) – L(L+1) – S(S+1)], A < 0 Reason: electrons with same spin need to have a wider spatial distribution (which according to Pauli’s principle correspond to different values m_l). The larger electron separations (less overlap, less repulsion) indeed lead to energetically more stable electronic configurations! (BE)
![Oxygen OI spinorbit states 3 P states S1 L1 fine structure lines 1 D Oxygen [OI] spin-orbit states 3 P states (S=1, L=1) fine structure lines 1 D](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-5.jpg)
Oxygen [OI] spin-orbit states 3 P states (S=1, L=1) fine structure lines 1 D states (S=0, L=2) no spin, no FSL 1 S states (S=0, L=0) no spin, no FSL oxygen p sub-shell is more than half full Hund’s rule then says 3 P 2 is ground state and 3 P 1 first excited state, 3 P 0 2 nd excited (the other way round for [OIII] and [CI])
![Oxygen OI multielectron system outer subshell 4 electrons 2 p4 Oxygen [OI] multi-electron system: outer sub-shell (4 electrons): 2 p^4](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-6.jpg)
Oxygen [OI] multi-electron system: outer sub-shell (4 electrons): 2 p^4
![OI fact sheet Ionisation potential 13 62 e V vs HI [OI] fact sheet • • • Ionisation potential: 13. 62 e. V (vs. HI](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-7.jpg)
[OI] fact sheet • • • Ionisation potential: 13. 62 e. V (vs. HI 13. 60 e. V) FSL lines: 63. 185 mu (3 P 1 -->3 P 2 gs), 145. 5 mu FSL energy levels: E = 228 K (3 P 1), 327 K (3 P 0) Excitation: collisions with electrons, H, H 2 High critical density: few x 10(5), few x 10(4) cm-3 for 3 P 1 and 3 P 0, respectively Def n_crit: collisional de-exc equals radiative de-exc assumption: optically thin case PS. Gas phase abundance: ~5 x 10(-4) of hydrogen
![Fact sheet CII CI and OIII CII 157 7 mu E Fact sheet [CII], [CI], and [OIII] • [CII] - 157. 7 mu, E =](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-8.jpg)
Fact sheet [CII], [CI], and [OIII] • [CII] - 157. 7 mu, E = 91. 2 K, IP([CI]) = 11. 3 e. V low n_crit ~=~ 2 x 10(3) cm-3 • [CI] - 609. 7 mu, 370. 4 mu; E = 23. 6 K, 62. 4 K n_crit = 620 cm-3, 720 cm-3 • [OIII] - 88. 4 mu, 51. 8 mu; E = 163 K , 441 K IP([OIII]) = 54. 9 e. V > I(He II, 54. 4 e. V)

Literature (text books) • B. T. Draine: Physics of ISM and IGM (2011) • Peter Bernath: spectra of atoms & molecules • Jonathan Tennyson: atoms in space • R. Genzel: Saas-Fee lectures (1991)
![Summary q The 63 mu 145 mu FSL lines of OI owe their Summary q The 63 mu / 145 mu FSL lines of [OI] owe their](https://slidetodoc.com/presentation_image/36d3d3415e5dfa4716042702ba276008/image-10.jpg)
Summary q The 63 mu / 145 mu FSL lines of [OI] owe their existence to quantum mech. (L = 1, S = 1 triplet, spin-orbit coupling) q Similar for [OIII] and [CI] – also FSL triplets. q [CII] S=1/2 FSL singlet (simplest case) q [CIII] S=0, closed shell, no FSL emission q Pauli’s exclusion principle and Hund’s rule 1 demonstrated in action for [OI] (L=0, 1, 2). What if the [OI] cooling lines did not exist?