The Quantum Mechanical Atom Bohrs Atom Planetary model

The Quantum Mechanical Atom

Bohr’s Atom • Planetary model predicted hydrogen emission spectrum exactly • Also worked for ions with only one electron (He+, Li+2) • Did not work for multi-electron atoms

de Broglie’s Wild Idea Maybe electrons act as waves! After all, light can act like a particle (photon). Momentum of a photon: p = h/l p = momentum h = Planck constant = 6. 626 10 -34 J s l = wavelength

de Broglie’s Wild Idea If electrons are waves, they can form standing waves when confined. Standing waves have specific shapes, frequencies, and energies. Hmmm…
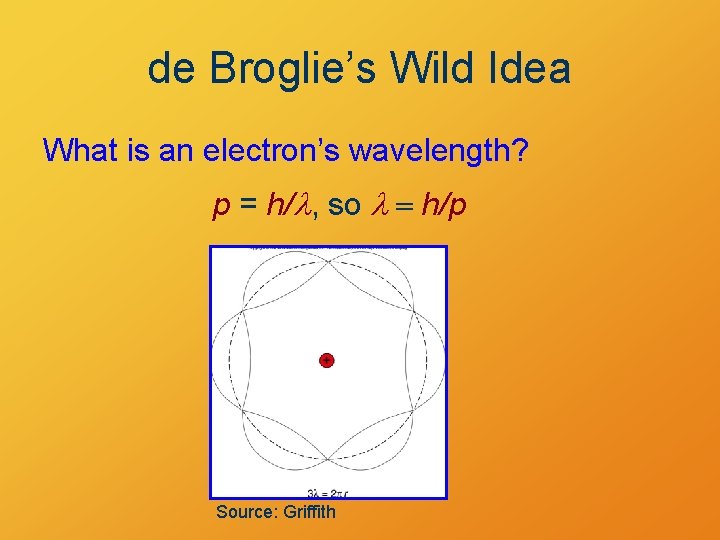
de Broglie’s Wild Idea What is an electron’s wavelength? p = h/l, so l = h/p Source: Griffith

Standing Waves Reminder • Confined waves can interfere with their reflections • Easy to see in one and two dimensions – Spring and slinky – Water surface – Membrane • Examples on next slide
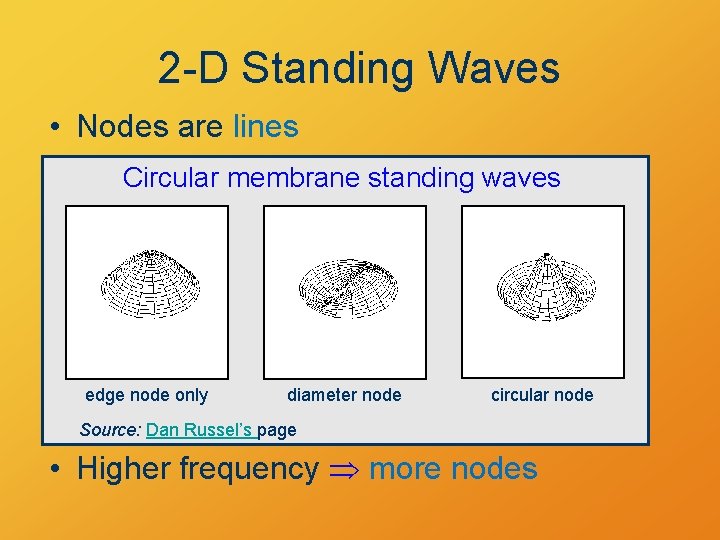
2 -D Standing Waves • Nodes are lines Circular membrane standing waves edge node only diameter node circular node Source: Dan Russel’s page • Higher frequency more nodes

Wavelength, frequency, and energy • Waves with short wavelengths have high frequency and high energy • Only short waves can fit in small spaces

Electron Energy Levels The electrons do not collapse onto the nucleus because: Smaller radius smaller wavelength This requires a larger energy! Lowest energy optimizes electric attraction and wavelength
Nuclei (Aside) • So… how can protons and neutrons be confined to a nucleus? • Momentum p = h/l, so wavelength l = h/p • Light, heavy objects with same l have same p § m. V = Mv • But… a heavy object has much lower speed and much lower kinetic energy! § ½ m. V 2 > ½ Mv 2 • So confined massive things can have lower KE

Quantum Wavefunctions • Tell everything we know about a particle • Mathematical functions of position & time • Determined by particle’s energy, mass, force fields • Square is probability density
Electron Orbitals • Higher energy more nodes • Exact shapes given by four quantum numbers § n, l, ml: wave shape (nodal pattern) § ms: electron “spin” • Pauli Exclusion Principle: No two electrons can have the same four quantum numbers
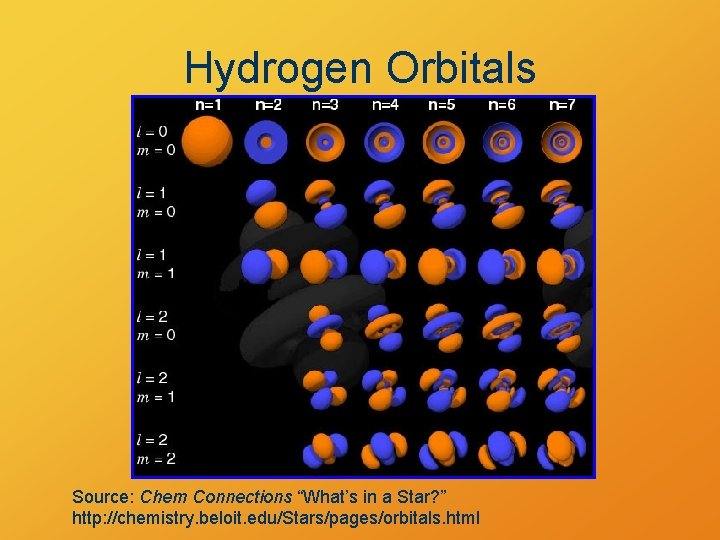
Hydrogen Orbitals Source: Chem Connections “What’s in a Star? ” http: //chemistry. beloit. edu/Stars/pages/orbitals. html

Filling Orbitals • An electron occupies the lowest-energy available orbital • If some orbitals have the same energy, occupy one at a time before pairing
Orbital Energies • Electron-electron repulsion makes multielectron atoms more complicated • For orbitals with the same number of total nodes, energy is higher for orbitals with more angular nodes
- Slides: 15