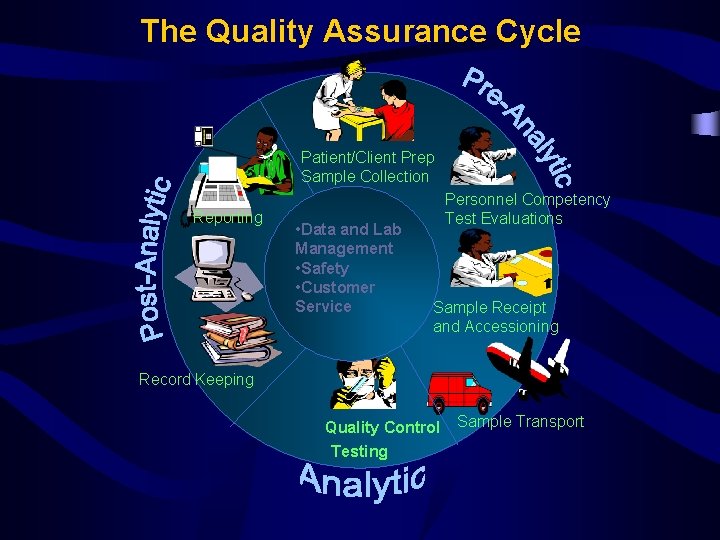
The Quality Assurance Cycle PatientClient Prep Sample Collection





























































- Slides: 70


The Quality Assurance Cycle Patient/Client Prep Sample Collection Reporting • Data and Lab Management • Safety • Customer Service Personnel Competency Test Evaluations Sample Receipt and Accessioning Record Keeping Quality Control Testing Sample Transport

• QA can be seen as the sum of QC, IQA and EQA QA = QC + IQA + EQA IQA: Internal quality assessment EQA: External quality assessment

Quality System Model In the laboratory

• Quality assurance (QA) – is the total process whereby the quality of laboratory reports can be guaranteed. • It has been summarized as the: right result, at the right time, on the right specimen, from the right patient, with the result interpretation based on correct reference data, and at the right price.

Attributes of a QA plan • Planned, systematic, and ongoing Comprehensive • Based on indicators and criteria with consensus approval • Routine surveillance and analysis of data • Documentation of problem identification and resolution • Continuous activity to ensure that quality performance is sustained • Integrated and interdepartmental

Quality and Improvement procedure • • Objectives Consensus Quality indicators Data analysis and strategies for improvement • Documentation

Quality management organization

Who is Responsible? • Lab Tech-The person who performs testing • Supervisor-The person who is responsible for day-to-day activities, training, delegation of work • Director-The person who is responsible for entire seamless operation, planning, and control of all activities • Ministry of Health-Place responsible for infrastructure, man power, and resources.

What is Quality Control? • Process or system for monitoring the quality of laboratory testing, and the accuracy and precision of results • Routinely collect and analyze data from every test run or procedure • Allows for immediate corrective action

• QC covers that part of QA, which primarily concerns the control of errors in the performance of tests and verification of test results • QC must cover all aspects of every procedure within the department • It must be practical, achievable, and affordable

Designing a QC Program – • Establish written Lab policies, Requisition forms, SOPs, Report forms, and Revisions and Corrective action plan • Assure complete documentation and review • Assure proper controls, standards, chemicals and storage • Equipment control and maintenance • Train all staff and periodic retraining • Periodic Internal audits

Qualitative vs. Quantitative • Qualitative test – determines whether the substance being tested for is present or absent • Quantitative test – measures the amount of a substance present

Qualitative QC • Quality control is performed for both, system is somewhat different • Controls available – Agglutination / precipitation controls : Blood Bank / Serology / Micro / Biochemistry / RPR/TPHA – Colour change: Dipstick technology, Pregnancy test Sterilization ampules, Occult blood, Biochemical reactions – Opacity tube standards: Mc. Farland std tubes

Specimen vs. Diagnosis

Quality assurance in Microbiology Lab • • • Quality control Standard operating procedures (SOPs) Pre-analytical stage Analytical stage Post-analytical stage Facilities Staff and qualifications External quality assessment (EQA) Internal quality assessment (IQA)

Pre-analytical stage-1 • Each specimen must be accompanied by a request form which details: a) Patient's name, age, gender, occupation, outpatient or inpatient number, ward or health center. b) Type and source of specimen, date and time of collection. c) Investigations required. d) Clinical note summarizing the patient's illness, suspected diagnosis and information on any antimicrobial treatment that may have been started at home or in the hospital.

Pre-analytical stage-2 • Collection and transport of specimens – the quality of the specimen submitted – timing of specimen – the suitability of sampling method and transport – use of transport media

Analytical stage -1 • The following should be incorporated in the microbiological SOPs covering the analytical stage: a) Detailed procedure for examining different specimens. b) Staining techniques and QC of stains. c) Aseptic techniques and safe handling of infectious material. d) Preparation and QC of culture media and preservation of stock strains.

Analytical stage • Fellow SOP • Control of stains and reagents • Control of Equipment

Analytical stage -2 e) Inoculation of liquid and solid media. f) Reading and interpretation of cultures. g) Techniques used to identify pathogens. h) Antimicrobial sensitivity testing and QC of procedures and antibiotic discs. i) Cleaning and QC of equipment used in microbiology laboratory.

Analytical stage -3 j) Immunologic techniques and QC of antigen and antibody reagents. k) Safe working practices. l) Disposal of specimens and cultures. m) Cleaning of glassware, plastic ware, etc. n) Sterilization procedures and their control.

Post-analytical stage 1. SOP needs to include reporting and verifying of microbiological test results, interpreting test reports correctly, taking appropriate action when a result has serious implications for a patient or public health. 2. Turn around time ─ no delay

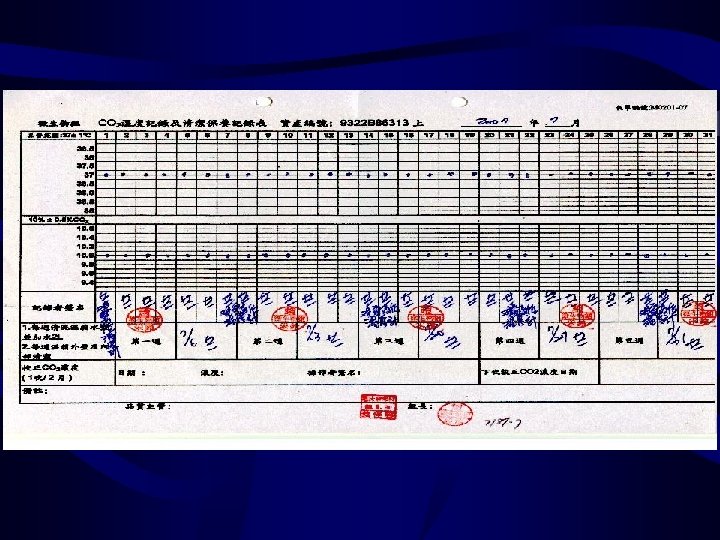
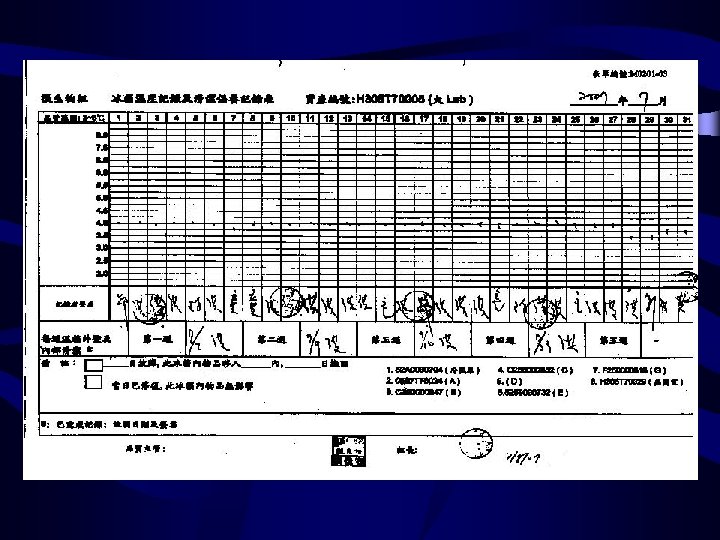
Equipment-1 the percentage of CO 2 temperature (2)BACTEC 9240 ---noninvasive fluorescent detection system 溫度 燈號 (3)Anaerobic chamber anaerobiasis humidity temperature the pressure of gas (4) Temperature Incubator (1)Incubator Refrigerator deep Freezer autoclave




Equipment-2 (5)Biosafety cabinet air flow (done by specialist) change HEPA filter UV light (6)Microscop (7) Centrifuge (8) VITEK clean adjust

Process and process improvement • Pre-analytical – Specimen collection, transport, receiving • Analytical – Culture media and reagent – Antimicrobial Susceptibility • Post-analytical – Preliminary result report – Critical result report and notification – Delayed result report

Process – Analytical • Culture media – Commercial media – In-house media

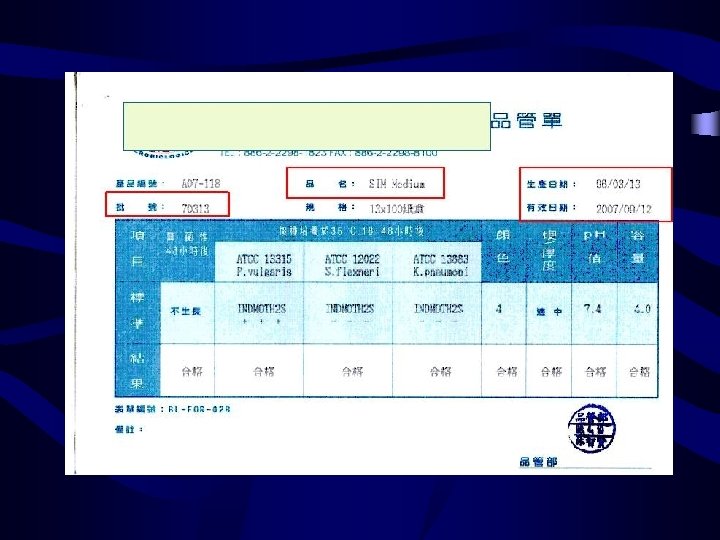
Process – Analytical • Media Preparation – Record amount – Lot number – Preparation date – Preparer – Expiration date

Process – Analytical • Commercial media – – Test by the manufacture 可不做sterility 及performance test 注意批次(lot) , 過期日期及是否污染等外觀 特殊功能培養基 : 如chocolate agar及selective media for pathogenic Neisseriae及CCFA必須每 一新批次皆要測試後才能用 – Mueller-Hinton agar, Mueller-Hinton BAP : 以 E. faecalis ATCC 29212測thiamine含量, SXT抑制圈應 >=20 mm




Process – Analytical Microbiology QC • Check: – Sterility – Ability to support growth – Selective or inhibitory characteristics of the medium – Biochemical response • Frequency – Test QC organisms with each new batch or lot number – Check for growth of fastidious organisms on media of choice –incubate at time and temp recommended – RECORD Results on Media QC form




Process – Analytical Stains, Reagents, Antisera • 1) 2) 3) 4) 5) 6) Label the containers Contents Concentration Date prepared Placed in service Expiration date/ shelf life preparer

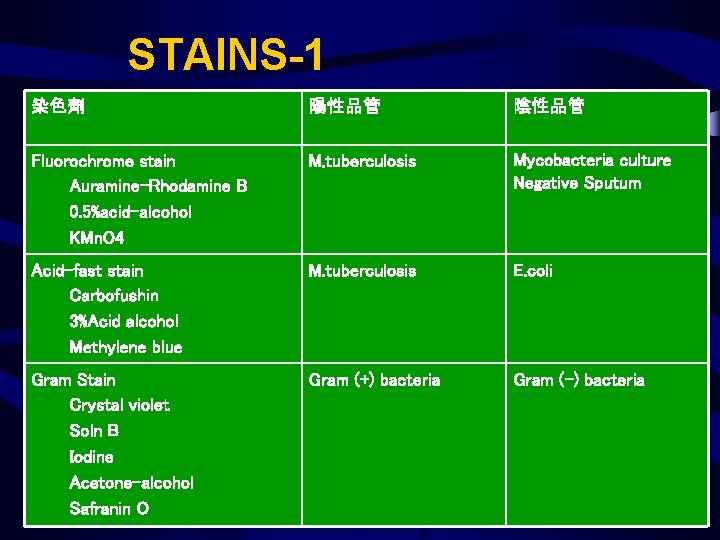
Process – Analytical Stains • • 每次新配製的染色試劑需記錄配製時間 Gram stain : 每週QC Acid-fast Stain : 每日QC Fluorochrome Stain : 每次QC

STAINS-1 染色劑 陽性品管 陰性品管 Fluorochrome stain Auramine-Rhodamine B 0. 5%acid-alcohol KMn. O 4 M. tuberculosis Mycobacteria culture Negative Sputum Acid-fast stain Carbofushin 3%Acid alcohol Methylene blue M. tuberculosis E. coli Gram Stain Crystal violet Soln B Iodine Acetone-alcohol Safranin O Gram (+) bacteria Gram (-) bacteria

泡製試劑 試劑 陽性品管 陰性品管 頻率 3%H 2 O 2 Sta. aureus Streptococcus 新Lot Coagulase Sta. aureus Sta. epidermidis 新Lot Oxidase Ps. aeruginosa E. coli 新Lot Indole(Kova c’s) E. coli Kleb. pneumonia 新Lot Spot indole E. coli Kleb. pneumonia 新Lot VP reagent E. cloacae E. coli 新Lot OR plate S. aureus ATCC S. aureus 43300 ATCC 29213 每批/每天 VA plate E. faecalis ATCC 51299 每批/每週 E. faecalis ATCC 29212 操作者

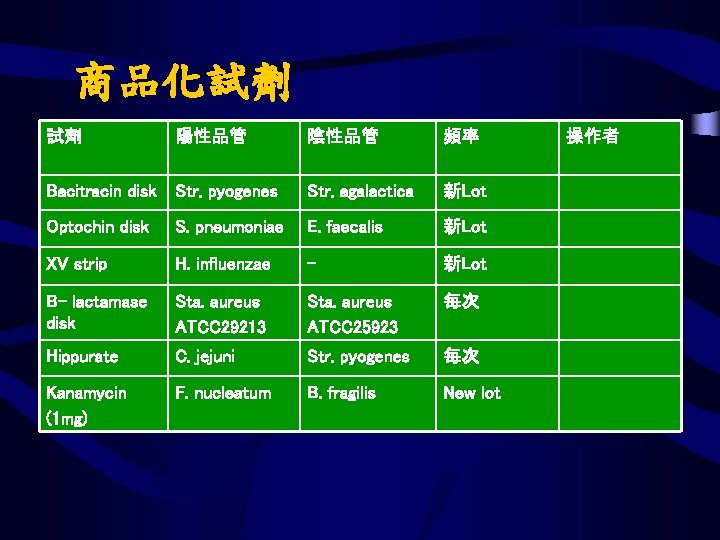
商品化試劑 試劑 陽性品管 陰性品管 頻率 Bacitracin disk Str. pyogenes Str. agalactica 新Lot Optochin disk S. pneumoniae E. faecalis 新Lot XV strip H. influenzae - 新Lot B- lactamase disk Sta. aureus ATCC 29213 Sta. aureus ATCC 25923 每次 Hippurate C. jejuni Str. pyogenes 每次 Kanamycin (1 mg) F. nucleatum B. fragilis New lot 操作者


商品化試劑-1 試劑 陽性品管 陰性品管 頻率 操作者 Vanomycin( 5 ug) Cl. perfringenes B. fragilis New lot AN table SPS disk P. anaerobius - New lot AN table Niacin strip M. tuberculosis NTM New lot TB table Novoboicin S. saprophyticus S. epidermidis 新Lot/每 週 Cover table CAZ/CLA, CTX/CLA disk K. pneumonia ATCC E. coli ATCC 25922 新Lot/每 週 Cover table O 129 disk V. cholera - 新Lot Cover table - 新Lot/每 週 Cover table E test strip( S. pneumonia Va, P)

抗血清試劑 (1)Salmonella , Shigella antiserum (2)S. pneumonia , H. influenzae b使用時 需同時操作藥組中所附之Positive control 及Negative control (3)Staphaurex plus(latex agglutination test) (4)品管頻率 : 開封時/每六個月

鑑定套組品管 試劑 陽性品管 頻率 操作者 API 20 E E. coli ATCC 25922 New lot Cover ID 32 GN Ps. aeruginosa ATCC 27853 C. albicans ATCC 90028 H. influenzae New lot Cover New lot Fungus Culture New lot Cover G. vaginalis New lot Cover ID 32 C API NH API CORYNE

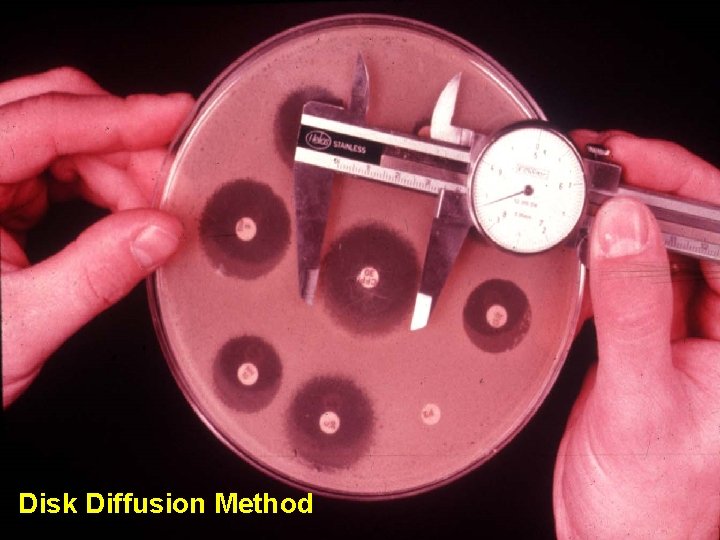
Antimicrobial Susceptibility Q. C (1)Disk Diffusion Method (2)MIC Method( Vitek system) (3) Two Endpoint-determining Method ( Agar Dilution Method ) (4)Agar Dilution Method

Disk Diffusion Method

MIC Determinations-Broth Macrodilution Method Control 1 g/ml 2 4 8 16 32 Antibiotic concentration 64 128 256

Broth Microdilution Method

Agar Dilution Method

Disk Diffusion Method-1 • 品管菌種 E. coli ATCC 25922 Sta. aureus ATCC 25923 Ps. aeruginosa ATCC 27853 H. influenzae ATCC 49247 Str. pneumonia ATCC 49619 E. faecalis ATCC 29212 ※標準菌種 : the specific strains from the American Type Collection(ATCC)



QC Procedure Flow Chart

Weekly QC FLOW Chart

MIC Method( Vitek system) • Susceptibility Card • Identification Card

VITEK 2 Advance d SYSTEM Expert System

Susceptibility Card • Q. C. 菌種 : E. coli ATCC 25922 Sta. aureus ATCC 29213 Ps. aeruginosa ATCC 27853 E. faecalis ATCC 29212 • Q. C. 頻率 : – New lot – weekly

Identification Card • Q. C. 菌種 : Sta. aureus ATCC 29213 Q. C. 頻率 : New lot

Agar Dilution Method • 結核菌藥敏試驗 • Q. C. 菌種 : CAP之測試菌種 • Q. C. 頻率 : a. new lot antimicrobial agents b. new lot media c. weekly

藥敏試驗品管記錄- Agar Dilution Method

Detecting Errors • Many organisms have predictable antimicrobial test results – Staphylococcus spp. are usually susceptible to vancomycin – Streptococcus pyogenes are always susceptible to penicillin – Klebsiella pneumoniae are resistant to ampicillin

Sources of Error • If you encounter an unusual pattern – rule out error by checking identification of organisms – repeat antimicrobial susceptibility test • Report if repeat testing yields same result, or refer the isolate to a reference laboratory for confirmation

Staff and qualifications • Graduate in medical laboratory technology. • continuing education program and inservice training


