The Pyrolysis of Methyl Acetate in a Heated

- Slides: 16

The Pyrolysis of Methyl Acetate in a Heated Micro-Reactor Jessie Porterfield June 22, 2017 72 nd Annual ISMS Porterfield JP, Bross DH, Ruscic B, Thorpe JH, Nguyen TL, Baraban JH, Stanton JF, Daily JW, Ellison GB, J. Phys. Chem. A. 2017 DOI: 10. 1021/acs. jpca. 7 b 02639

Motivation - methyl esters as biodiesel § Biofuels can reduce anthropogenic emissions of CO 2 § Transportation accounts for ~ ¼ total energy, existing infrastructure requires liquid fuel (automobiles, ships, aircrafts) § Domestic airline fuel consumption in 2015 alone was 4 x 1010 liters – must replace with something abundant § Esters can be derived from the membranes of biological species § Initial radical formation in an engine causes a cascade of chemistry – combustion efficiency, emissions Jessie Porterfield The Pyrolysis of Methyl Acetate 72 nd Annual ISMS

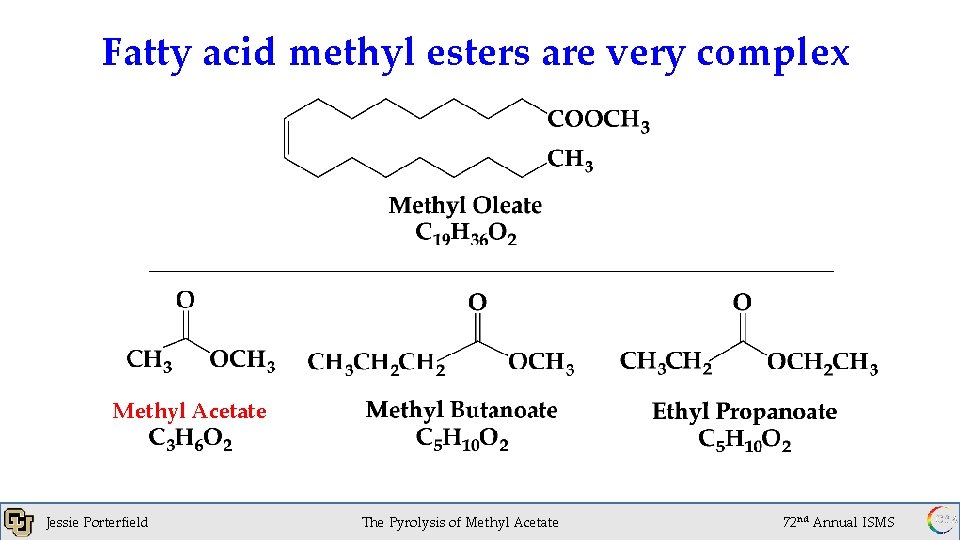
Fatty acid methyl esters are very complex Methyl Acetate Jessie Porterfield The Pyrolysis of Methyl Acetate 72 nd Annual ISMS

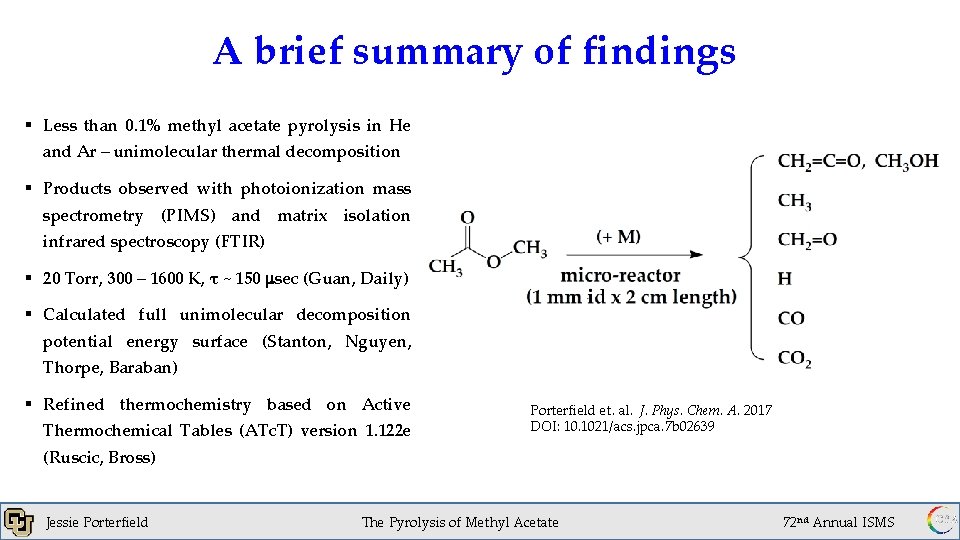
A brief summary of findings § Less than 0. 1% methyl acetate pyrolysis in He and Ar – unimolecular thermal decomposition § Products observed with photoionization mass spectrometry (PIMS) and matrix isolation infrared spectroscopy (FTIR) § 20 Torr, 300 – 1600 K, τ ~ 150 msec (Guan, Daily) § Calculated full unimolecular decomposition potential energy surface (Stanton, Nguyen, Thorpe, Baraban) § Refined thermochemistry based on Active Thermochemical Tables (ATc. T) version 1. 122 e Porterfield et. al. J. Phys. Chem. A. 2017 DOI: 10. 1021/acs. jpca. 7 b 02639 (Ruscic, Bross) Jessie Porterfield The Pyrolysis of Methyl Acetate 72 nd Annual ISMS

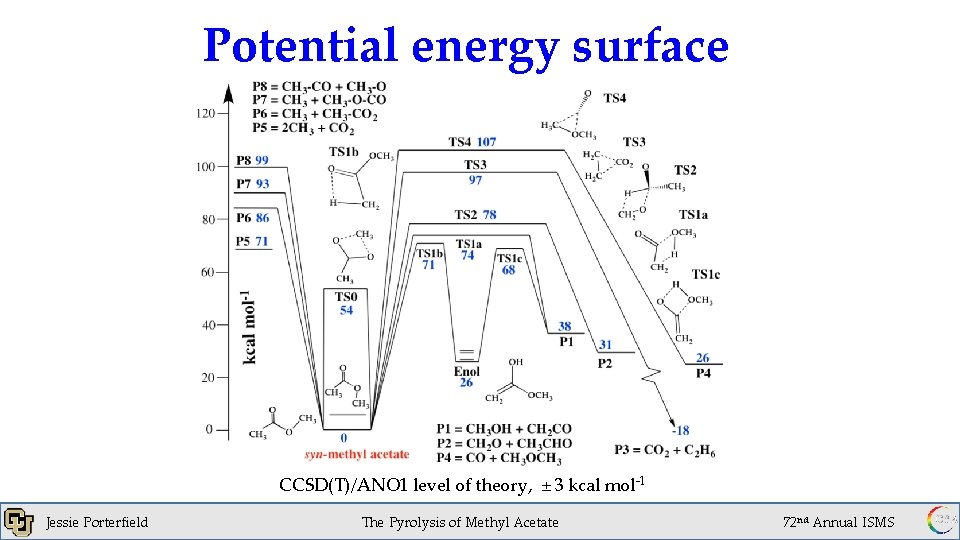
Potential energy surface CCSD(T)/ANO 1 level of theory, ± 3 kcal mol-1 Jessie Porterfield The Pyrolysis of Methyl Acetate 72 nd Annual ISMS

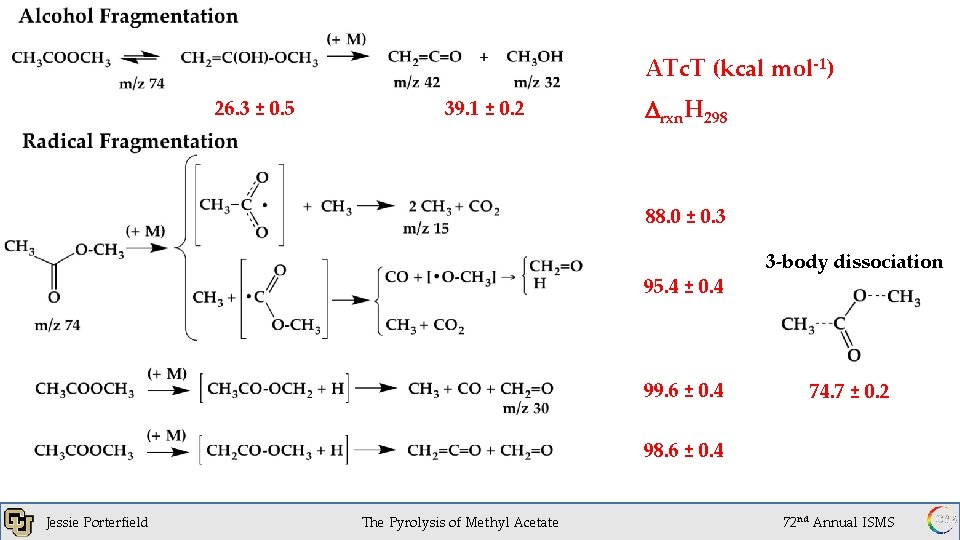
ATc. T (kcal mol-1) 26. 3 ± 0. 5 39. 1 ± 0. 2 Drxn. H 298 88. 0 ± 0. 3 95. 4 ± 0. 4 99. 6 ± 0. 4 3 -body dissociation 74. 7 ± 0. 2 98. 6 ± 0. 4 Jessie Porterfield 1 x 10 of. Torr The Pyrolysis Methyl Acetate -6 72 nd Annual ISMS

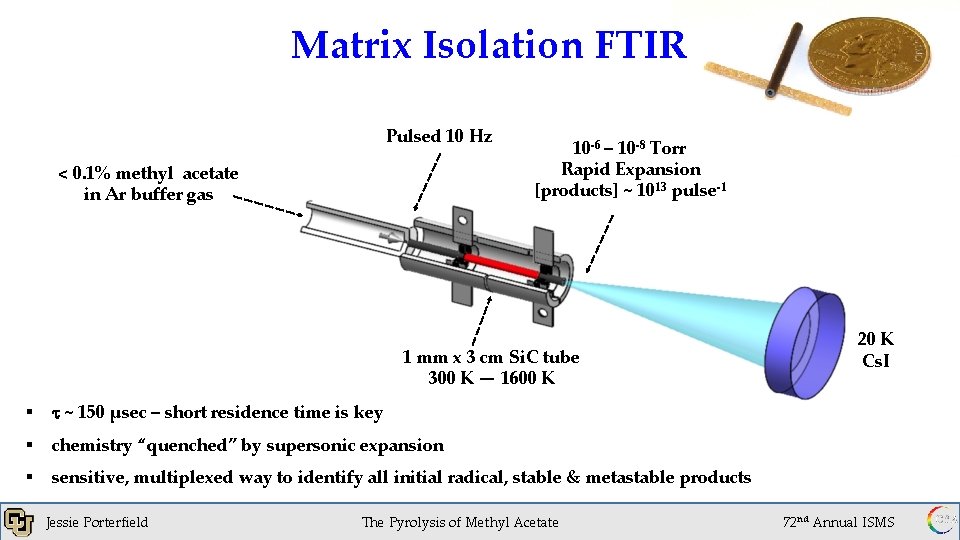
Matrix Isolation FTIR Pulsed 10 Hz < 0. 1% methyl acetate in Ar buffer gas 10 -6 – 10 -8 Torr Rapid Expansion [products] ~ 1013 pulse-1 1 mm x 3 cm Si. C tube 300 K — 1600 K § t ~ 150 µsec – short residence time is key § chemistry “quenched” by supersonic expansion § sensitive, multiplexed way to identify all initial radical, stable & metastable products Jessie Porterfield The Pyrolysis of Methyl Acetate 20 K Cs. I 72 nd Annual ISMS

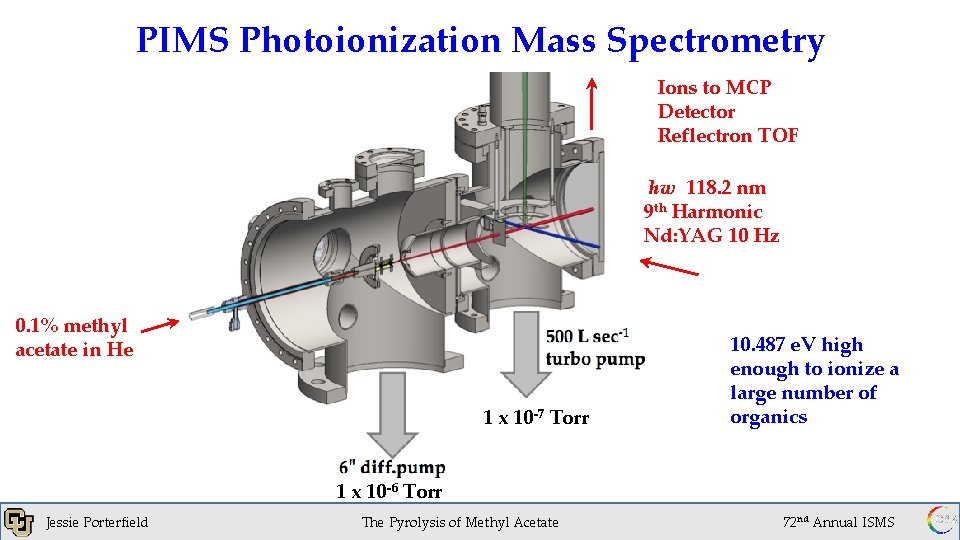
PIMS Photoionization Mass Spectrometry Ions to MCP Detector Reflectron TOF hw 118. 2 nm 9 th Harmonic Nd: YAG 10 Hz 0. 1% methyl acetate in He 1 x 10 -7 Torr Jessie Porterfield 1 x 10 -6 Torr 1 x 10 -6 of. Torr The Pyrolysis Methyl Acetate 10. 487 e. V high enough to ionize a large number of organics 72 nd Annual ISMS

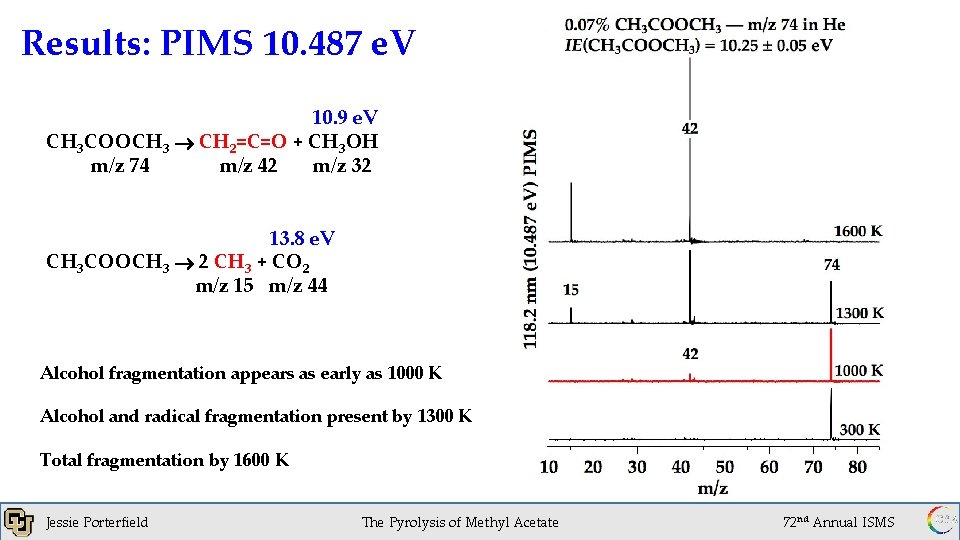
Results: PIMS 10. 487 e. V 10. 9 e. V CH 3 COOCH 3 CH 2=C=O + CH 3 OH m/z 74 m/z 42 m/z 32 13. 8 e. V CH 3 COOCH 3 2 CH 3 + CO 2 m/z 15 m/z 44 Alcohol fragmentation appears as early as 1000 K Alcohol and radical fragmentation present by 1300 K Total fragmentation by 1600 K Jessie Porterfield 1 x 10 of. Torr The Pyrolysis Methyl Acetate -6 72 nd Annual ISMS

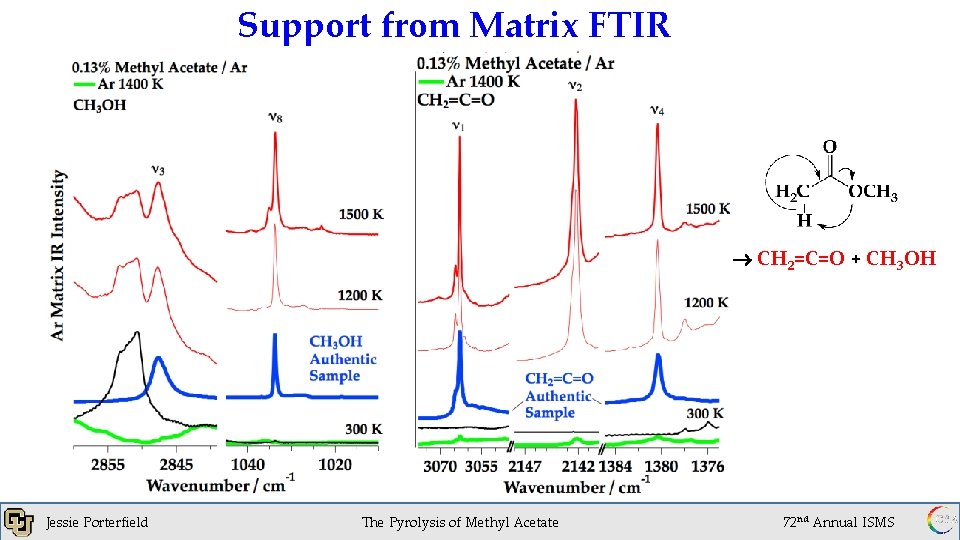
Support from Matrix FTIR CH 2=C=O + CH 3 OH Jessie Porterfield 1 x 10 of. Torr The Pyrolysis Methyl Acetate -6 72 nd Annual ISMS

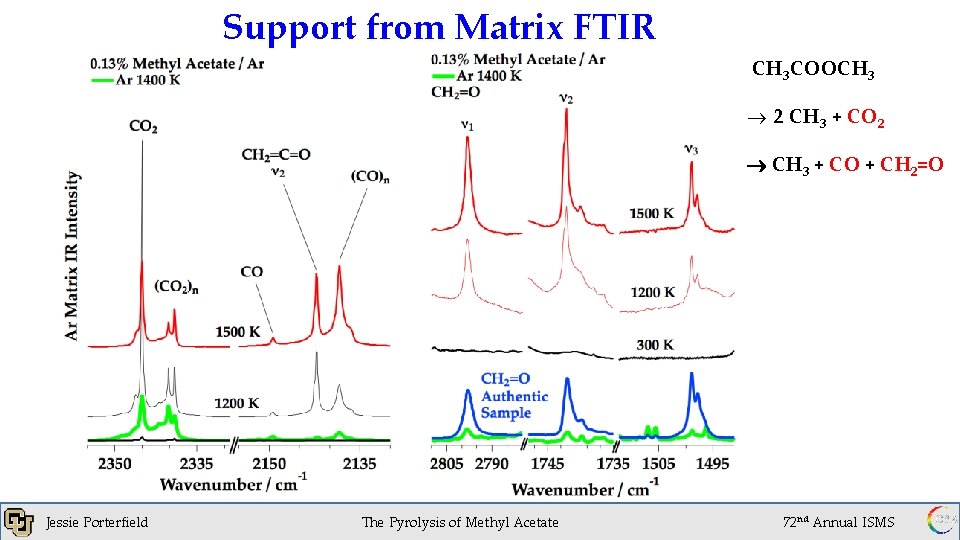
Support from Matrix FTIR CH 3 COOCH 3 ® 2 CH 3 + CO 2 CH 3 + CO + CH 2=O Jessie Porterfield 1 x 10 of. Torr The Pyrolysis Methyl Acetate -6 72 nd Annual ISMS

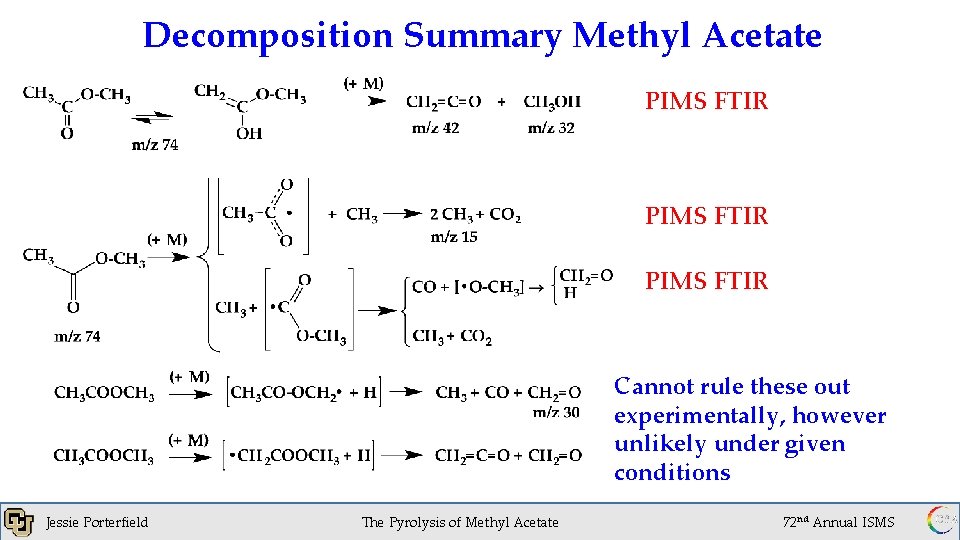
Decomposition Summary Methyl Acetate PIMS FTIR Cannot rule these out experimentally, however unlikely under given conditions Jessie Porterfield 1 x 10 of. Torr The Pyrolysis Methyl Acetate -6 72 nd Annual ISMS

Acknowledgements Harvard Smithsonian Center for Astrophysics Michael Mc. Carthy Carl Gottlieb University of Colorado at Boulder G. Barney Ellison Josh Baraban John W. Daily University of Texas, Austin John Stanton James Thorpe Thanh Lam Nguyen Argonne National Labs David H. Bross Branko Ruscic JPorterfield@cfa. harvard. edu Porterfield JP, Bross DH, Ruscic B, Thorpe JH, Nguyen TL, Baraban JH, Stanton JF, Daily JW, Ellison GB, J. Phys. Chem. A. 2017 DOI: 10. 1021/acs. jpca. 7 b 02639 Jessie Porterfield 1 x 10 of. Torr The Pyrolysis Methyl Acetate -6 72 nd Annual ISMS

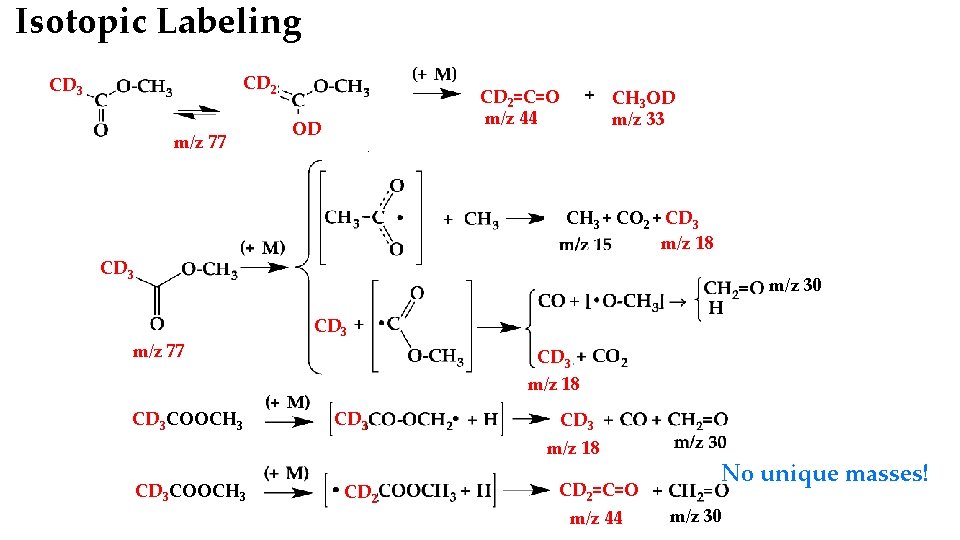
Isotopic Labeling CD 2 CD 3 m/z 77 CD 2=C=O m/z 44 OD CH 3 OD m/z 33 CH 3 + CO 2 + CD 3 m/z 18 CD 3 m/z 30 m/z 77 CD 3 COOCH 3 CD 3 m/z 18 CD 3 CD 2 CD 3 m/z 18 15 m/z CD 2=C=O m/z 44 No unique masses! m/z 30

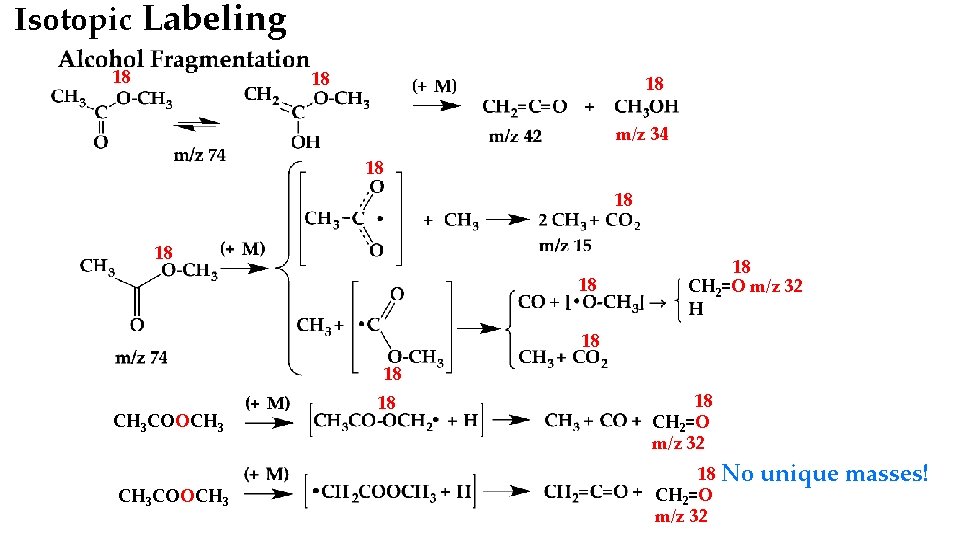
Isotopic Labeling 18 18 18 m/z 34 18 18 18 CH 2=O m/z 32 18 18 CH 3 COOCH 3 18 18 CH 2=O m/z 32 No unique masses!

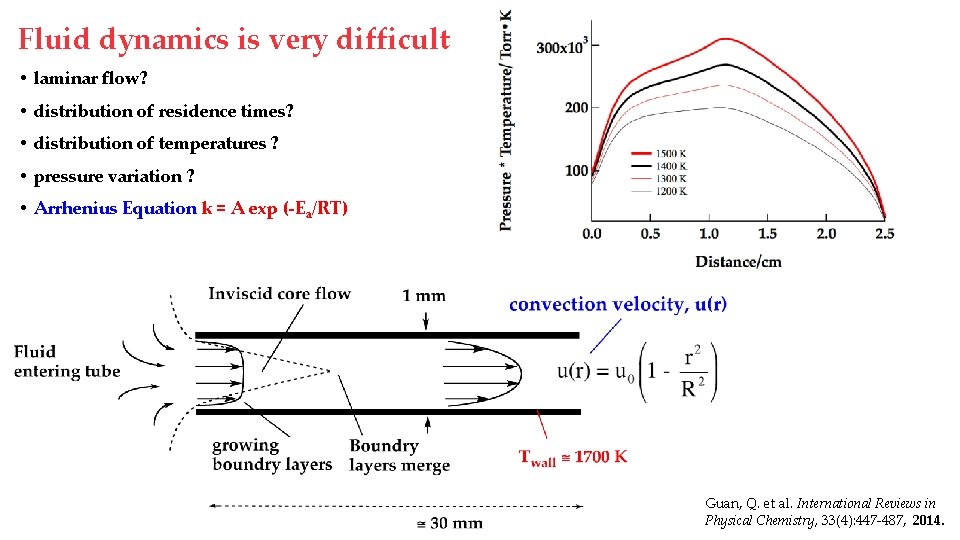
Fluid dynamics is very difficult • laminar flow? • distribution of residence times? • distribution of temperatures ? • pressure variation ? • Arrhenius Equation k = A exp (-Ea/RT) Guan, Q. et al. International Reviews in Physical Chemistry, 33(4): 447 -487, 2014.