The Prostate Net Prostate Cancer Symposium Inaugural Meeting



















- Slides: 41

The Prostate Net Prostate Cancer Symposium Inaugural Meeting New York City October 6, 2009 Emerging Treatments on the Horizon Celestia S. Higano MD, FACP Professor Medicine and Urology University of Washington Member, Fred Hutchison Cancer Research Center

Many Potential New Therapeutic Agents on the Horizon § AR targeted hormonal therapy Abiraterone – MDV 3100 – § Immunotherapy Provenge – Ipilimumab – § Other emerging therapies

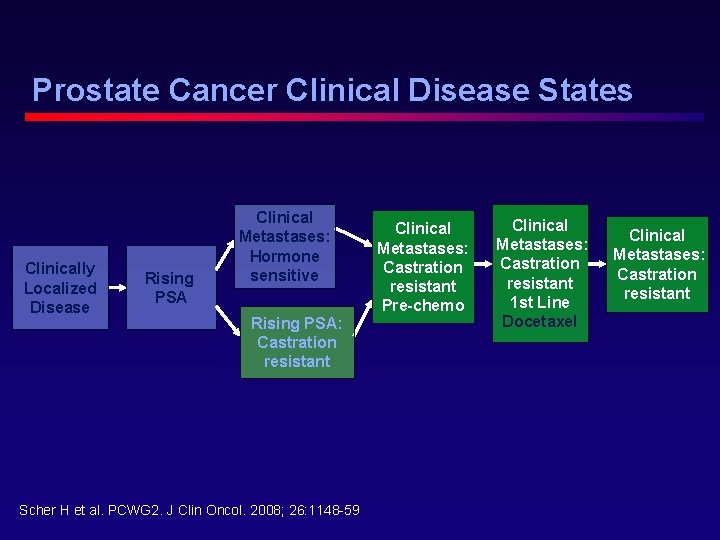
Prostate Cancer Clinical Disease States Clinically Localized Disease Rising PSA Clinical Metastases: Hormone sensitive Rising PSA: Castration resistant Scher H et al. PCWG 2. J Clin Oncol. 2008; 26: 1148 -59. Clinical Metastases: Castration resistant Pre-chemo Clinical Metastases: Castration resistant 1 st Line Docetaxel Clinical Metastases: Castration resistant

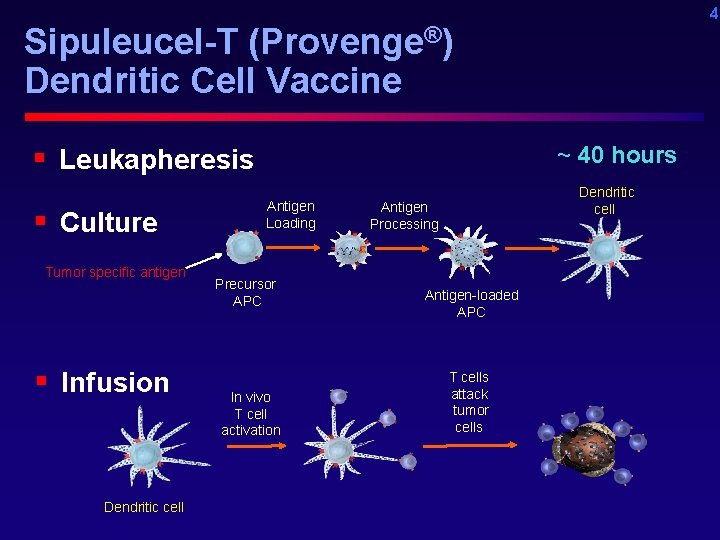
4 Sipuleucel-T (Provenge®) Dendritic Cell Vaccine § Leukapheresis § Culture Tumor specific antigen § Infusion Dendritic cell ~ 40 hours Antigen Loading Precursor APC In vivo T cell activation Dendritic cell Antigen Processing Antigen-loaded APC T cells attack tumor cells

Phase III IMPACT Trial P R O G R E S S I O N Treated at Physician discretion Sipuleucel. Asymptomatic T or Minimally Q 2 weeks Symptomatic 2: 1 x 3 Metastatic Treated at Castrate Physician Placebo discretion Resistant and/or Salvage Q 2 weeks Prostate Protocol 3 x Cancer (N=512) Primary. Preendpoint: Overall Survival chemotherapy Secondary endpoint: Time to Objective Disease Progression S U R V I V A L

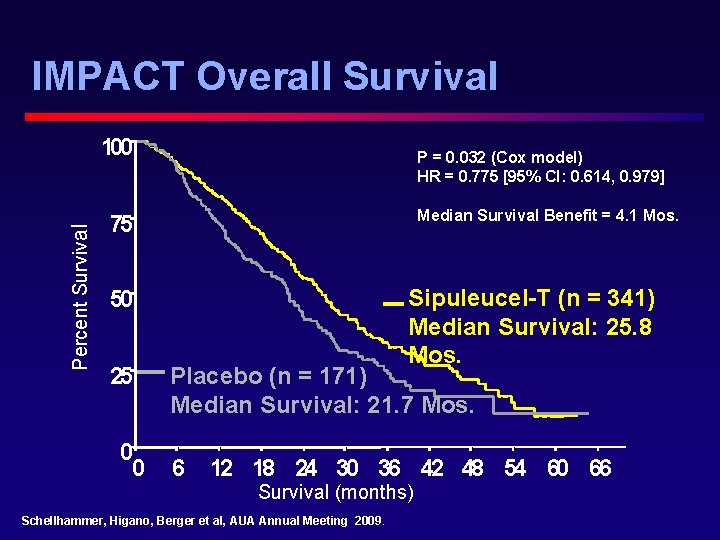
IMPACT Overall Survival Percent Survival 100 P = 0. 032 (Cox model) HR = 0. 775 [95% CI: 0. 614, 0. 979] Median Survival Benefit = 4. 1 Mos. 75 Sipuleucel-T (n = 341) Median Survival: 25. 8 Mos. 50 25 0 0 Placebo (n = 171) Median Survival: 21. 7 Mos. 6 12 18 24 30 36 42 48 54 60 66 Survival (months) Schellhammer, Higano, Berger et al, AUA Annual Meeting 2009.

Toxicity § Chills § Fever § Headache

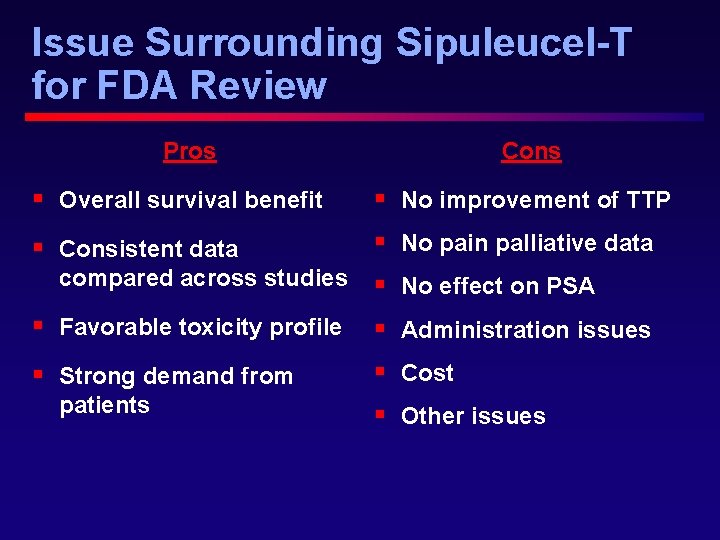
Issue Surrounding Sipuleucel-T for FDA Review Pros Cons § Overall survival benefit § No improvement of TTP § Consistent data § No pain palliative data compared across studies § No effect on PSA § Favorable toxicity profile § Administration issues § Strong demand from § Cost patients § Other issues

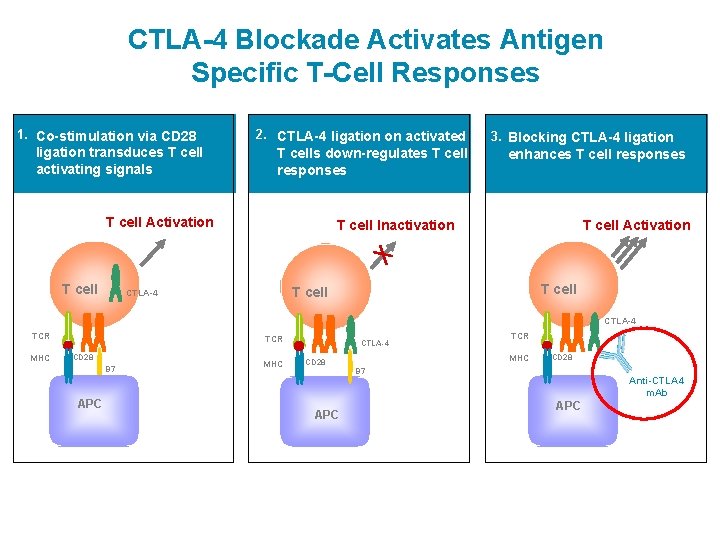
CTLA-4 Blockade Activates Antigen Specific T-Cell Responses 1. Co-stimulation via CD 28 ligation transduces T cell activating signals 2. CTLA-4 ligation on activated T cells down-regulates T cell responses T cell Activation T cell 3. Blocking CTLA-4 ligation enhances T cell responses T cell Inactivation T cell Activation T cell CTLA-4 TCR MHC TCR CD 28 B 7 APC MHC CTLA-4 TCR MHC CD 28 B 7 APC Anti-CTLA 4 m. Ab APC

1 0 Ipilimumab (Anti-CTLA 4 antibody) § Phase II Ipi alone and with single dose radiation § Phase III trial § Ipi and GVAX

Radiation promotes cross-presentation of tumor associated antigens Anti-CTLA 4 m. Ab CTLA-4 Modified after: Demaria, et al, Int. J. Radiation Oncology Biol. Phys. , Vol. 63, No. 3, pp. 655– 666, 2005

Ipilimumab Phase II 1 st Treatment Cycle Dosing q 3 wk x 4 Screening Follow Up or Additional Treatment Cycle(s); Assessments q 3 mos XRT -28 -2 1 IPI 22 IPI 43 IPI 64 IPI 421 (Days) (14 months) Or until PD 85 Cohort 1 Mono Cohort 2 XRT in No. CHEMO Cohort 3 XRT in CHEMO 10 mg/kg IPI XRT + 10 mg/kg IPI Chemo naïve n = 16 XRT + 10 mg/kg IPI Chemo experienced n = 18 n =16 Slovin, Beer, Higano et al GU ASCO 2009

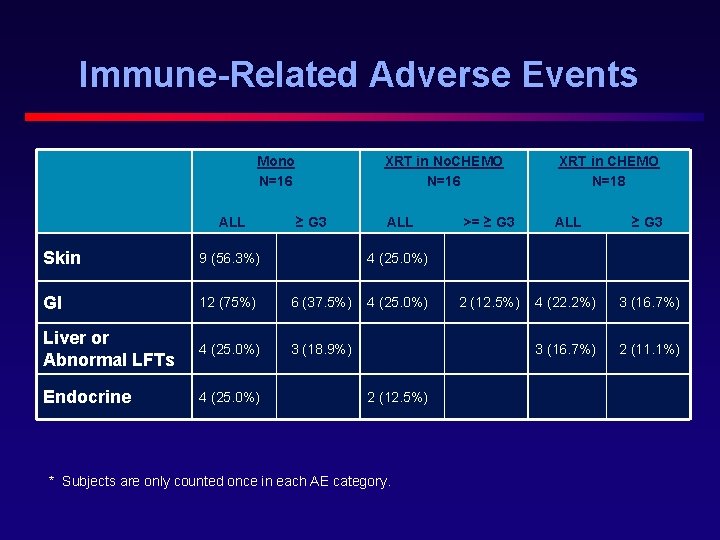
Immune-Related Adverse Events Mono N=16 ALL XRT in No. CHEMO N=16 ≥ G 3 Skin 9 (56. 3%) GI 12 (75%) 6 (37. 5%) Liver or Abnormal LFTs 4 (25. 0%) 3 (18. 9%) Endocrine 4 (25. 0%) ALL XRT in CHEMO N=18 >= ≥ G 3 ALL ≥ G 3 2 (12. 5%) 4 (22. 2%) 3 (16. 7%) 2 (11. 1%) 4 (25. 0%) 2 (12. 5%) * Subjects are only counted once in each AE category.

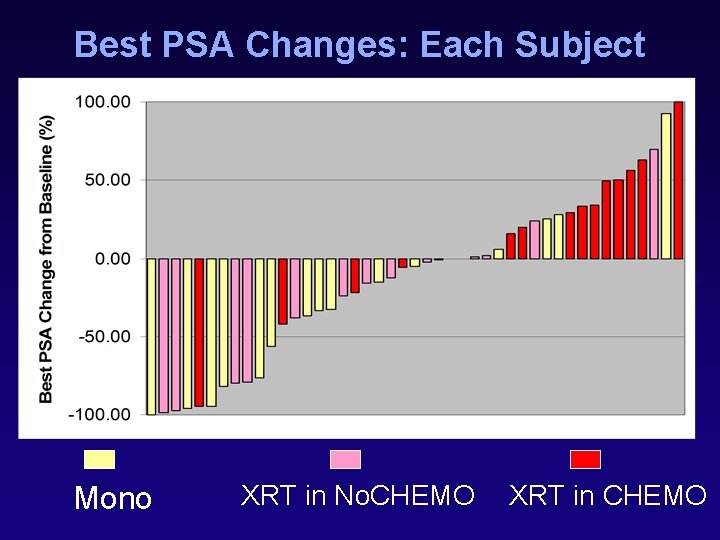
Best PSA Changes: Each Subject Mono XRT in No. CHEMO XRT in CHEMO

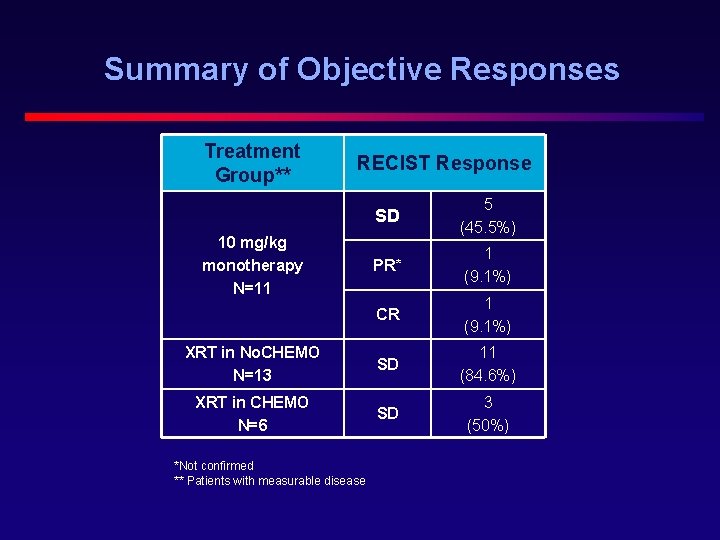
Summary of Objective Responses Treatment Group** RECIST Response SD 5 (45. 5%) PR* 1 (9. 1%) CR 1 (9. 1%) XRT in No. CHEMO N=13 SD 11 (84. 6%) XRT in CHEMO N=6 SD 3 (50%) 10 mg/kg monotherapy N=11 *Not confirmed ** Patients with measurable disease

Phase III Trial of Radiation plus Placebo or Ipilimumab § Must have received prior docetaxel § No more than 2 prior chemotherapy regimens § At least one bone lesion that has not been irradiated § Not eligible Men with auto-immune diseases – Prior treatment with strontium or samarium – 1 6

1 7 Where is immunotherapy going? § Provenge likely to be approved – Who should get it? § GVAX is dead for now – Survival data for Vital 1 in progress in Seattle § Ipililumab just starting phase III Can this type of agent be used in the community? – Stand by… –

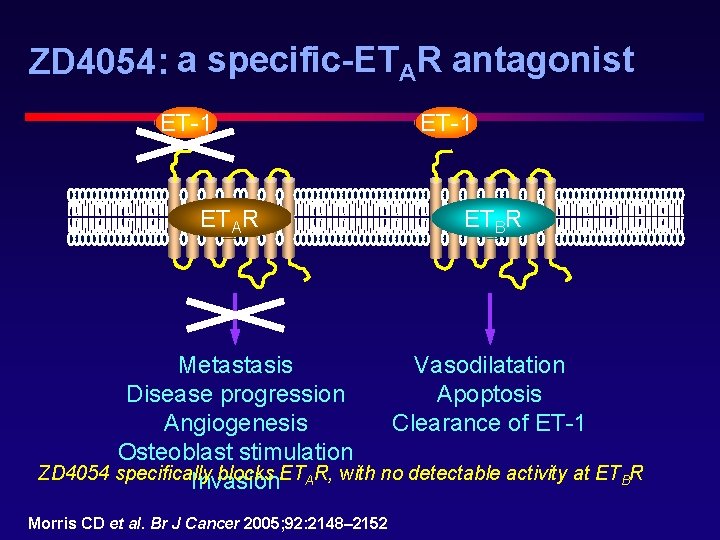
ZD 4054: a specific-ETAR antagonist ET-1 ETAR ET-1 ETBR Vasodilatation Metastasis Apoptosis Disease progression Clearance of ET-1 Angiogenesis Osteoblast stimulation ZD 4054 specifically blocks ETAR, with no detectable activity at ETBR Invasion Morris CD et al. Br J Cancer 2005; 92: 2148– 2152

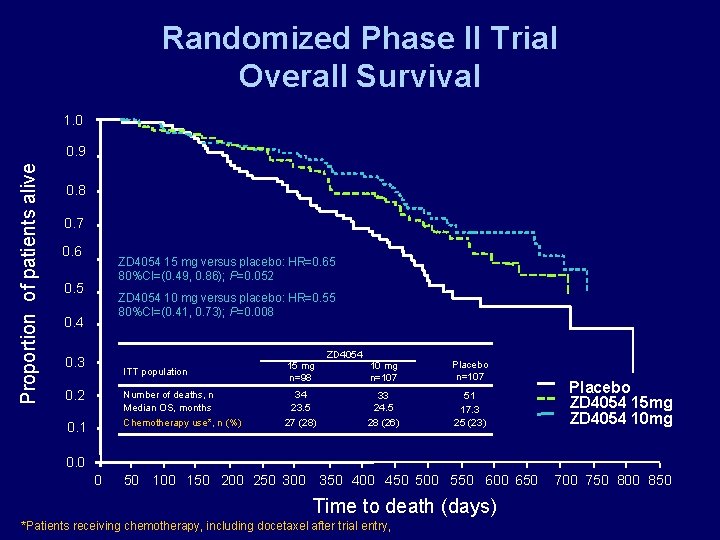
Randomized Phase II Trial Overall Survival 1. 0 Proportion of patients alive 0. 9 0. 8 0. 7 0. 6 ZD 4054 15 mg versus placebo: HR=0. 65 80%CI=(0. 49, 0. 86); P=0. 052 0. 5 ZD 4054 10 mg versus placebo: HR=0. 55 80%CI=(0. 41, 0. 73); P=0. 008 0. 4 0. 3 0. 2 0. 1 ITT population 15 mg n=98 Number of deaths, n Median OS, months Chemotherapy use*, n (%) 34 23. 5 27 (28) ZD 4054 10 mg n=107 Placebo n=107 33 24. 5 28 (26) 51 17. 3 25 (23) Placebo ZD 4054 15 mg ZD 4054 10 mg 0. 0 0 50 100 150 200 250 300 350 400 450 500 550 600 650 Time to death (days) *Patients receiving chemotherapy, including docetaxel after trial entry, 700 750 800 850

2 0 Enthuse Phase III Trials § Non-metastatic CRPC – 531 of 1500 § Asymptomatic metastatic CRPC Fully accrued, n=580 – Results Q 2 2010, 2011 ASCO – § Symptomatic metastatic CRPC – 591 of 1044

2 1 Other On-going Phase III Trials in CRPC § CALGB docetaxel +/- bevacizumab (Avastin®) – Results 2010 ASCO § SWOG docetaxel +/- atrasentan – 694 of 930 accrued § Docetaxel +/- VEGF Trap – 800 of 1200 accrued § Docetaxel +/- ZD 4054 – 591 of 1044

2 2 Take Home Messages § More active drugs § Many on-going and planned registration trials § Lots of potential! § Need patient participation to make progress!

? ARS

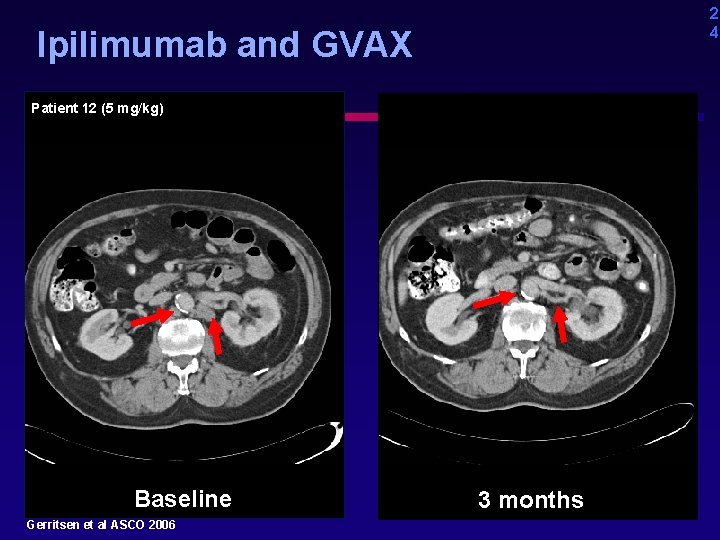
2 4 Ipilimumab and GVAX Patient 12 (5 mg/kg) Baseline Gerritsen et al ASCO 2006 3 months

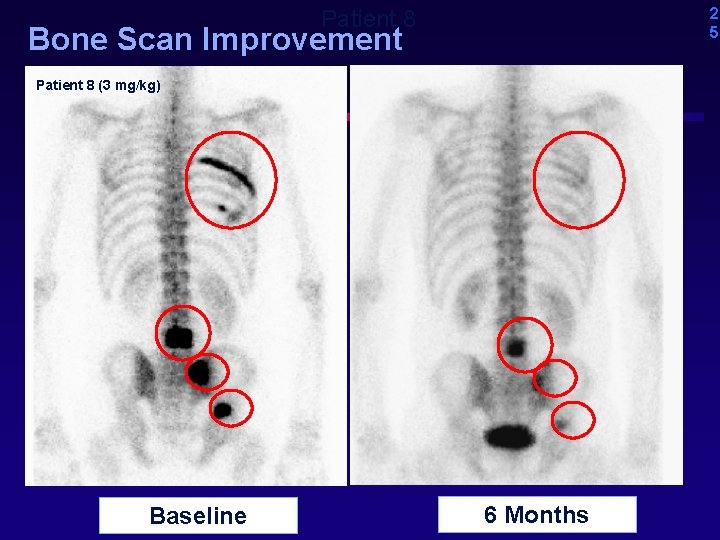
2 5 Patient 8 Bone Scan Improvement Patient 8 (3 mg/kg) Baseline 6 Months

2 6 GVAX Cellular Vaccine LNCa. P PC 3 DC Ward et al. Cancer Immunol Immunother. 2002: 51: 351. Antigenloaded DC

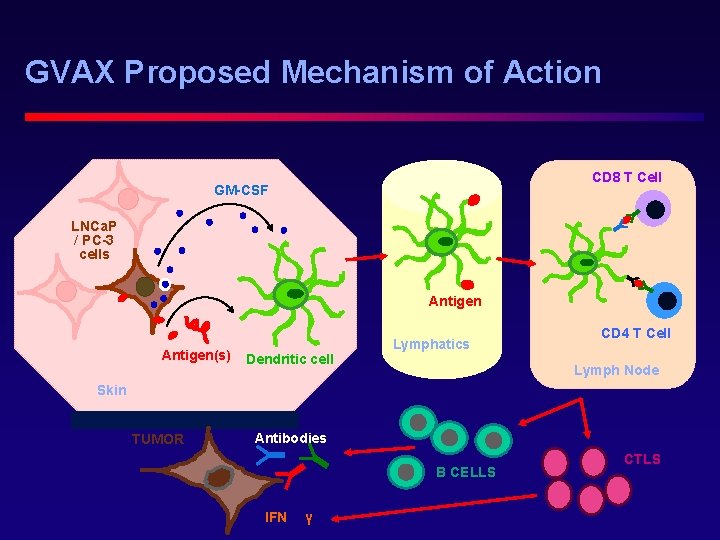
GVAX Proposed Mechanism of Action CD 8 T Cell GM-CSF LNCa. P / PC-3 cells Antigen(s) Dendritic cell Lymphatics CD 4 T Cell Lymph Node Skin TUMOR Antibodies B CELLS IFN γ CTLS

2 8 GVAX Immunotherapy § 6 injections each leg or arm (12 injections each time) § Treatments every 3 weeks § Injection site redness and itching Data on file, Cell Genesys, Inc.

2 9 GVAX Phase III Trials § Vital 1 § Vital 2

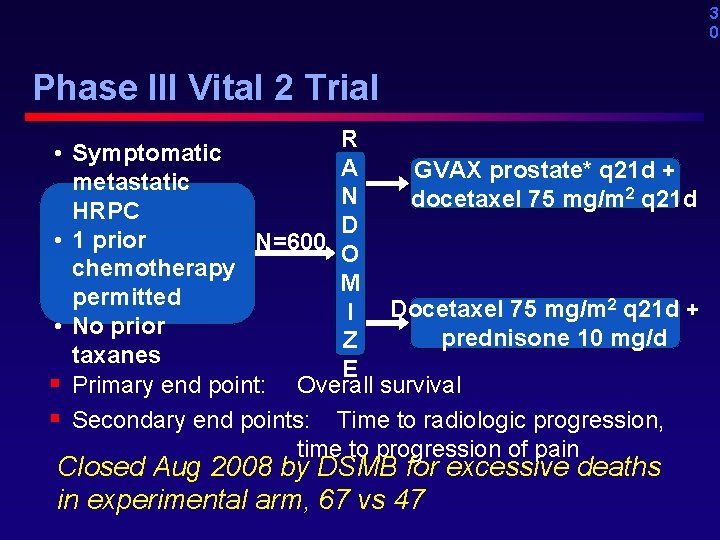
3 0 Phase III Vital 2 Trial • • • § § R Symptomatic A GVAX prostate* q 21 d + metastatic N docetaxel 75 mg/m 2 q 21 d HRPC D 1 prior N=600 O chemotherapy M permitted Docetaxel 75 mg/m 2 q 21 d + I No prior prednisone 10 mg/d Z taxanes E Primary end point: Overall survival Secondary end points: Time to radiologic progression, time to progression of pain Closed Aug 2008 by DSMB for excessive deaths in experimental arm, 67 vs 47

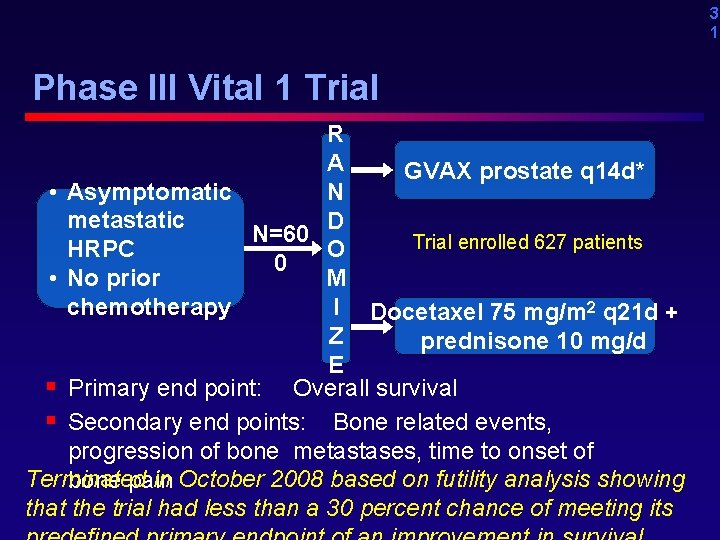
3 1 Phase III Vital 1 Trial R A GVAX prostate q 14 d* • Asymptomatic N metastatic D N=60 Trial enrolled 627 patients HRPC O 0 • No prior M chemotherapy I Docetaxel 75 mg/m 2 q 21 d + Z prednisone 10 mg/d E § Primary end point: Overall survival § Secondary end points: Bone related events, progression of bone metastases, time to onset of Terminated in October 2008 based on futility analysis showing bone pain that the trial had less than a 30 percent chance of meeting its

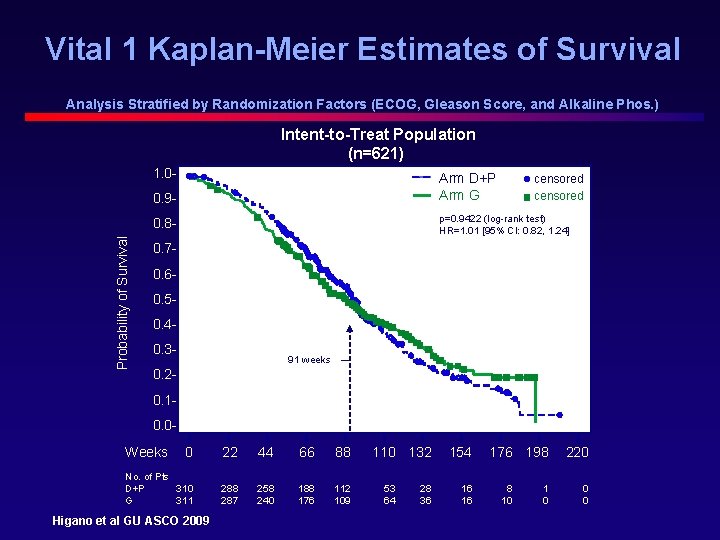
Vital 1 Kaplan-Meier Estimates of Survival Analysis Stratified by Randomization Factors (ECOG, Gleason Score, and Alkaline Phos. ) Intent-to-Treat Population (n=621) 1. 0 - Arm D+P Arm G 0. 9 - p=0. 9422 (log-rank test) HR=1. 01 [95% CI: 0. 82, 1. 24] 0. 8 Probability of Survival censored 0. 70. 60. 50. 40. 3 - 91 weeks 0. 20. 10. 0 - Weeks 0 22 44 66 88 No. of Pts D+P 310 G 311 288 287 258 240 188 176 112 109 Higano et al GU ASCO 2009 110 132 53 64 28 36 154 16 16 176 198 8 10 1 0 220 0 0

Vital 1 Kaplan-Meier Estimates of Survival Analysis Stratified by Randomization Factors (ECOG, Gleason Score, and Alkaline Phos. ) Patients with a Halabi Predicted Survival Time of ≥ 18 months (n=264) 1. 0 - Arm D+P Arm G 0. 9 Probability of Survival 0. 8 - censored HR= 0. 90 [95% CI: 0. 61, 1. 33] 0. 70. 60. 50. 40. 3 - 92 weeks 0. 20. 10. 0ɩ 0 Higano et al GU ASCO 2009 ɩ 22 ɩ 44 ɩ 66 ɩ ɩ ɩ ɩ 88 110 132 154 176 198 220

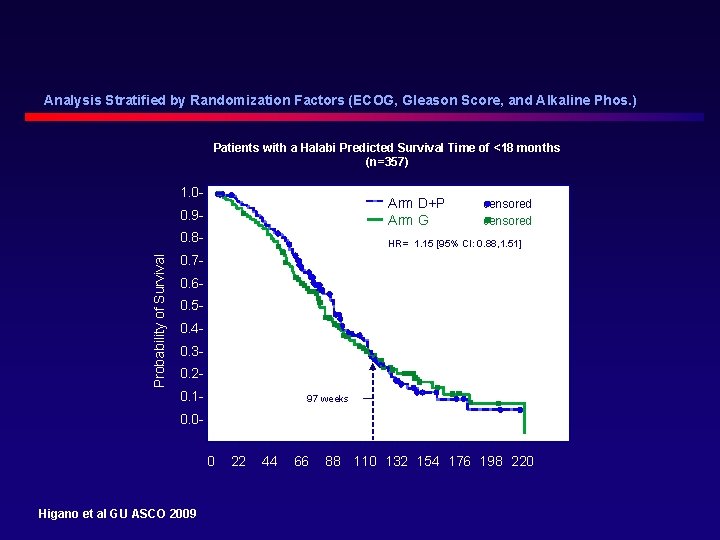
Analysis Stratified by Randomization Factors (ECOG, Gleason Score, and Alkaline Phos. ) Patients with a Halabi Predicted Survival Time of <18 months (n=357) Probability of Survival 1. 00. 9 - Arm D+P Arm G 0. 8 - HR= 1. 15 [95% CI: 0. 88, 1. 51] censored 0. 70. 60. 50. 40. 30. 20. 1 - 97 weeks 0. 0ɩ 0 Higano et al GU ASCO 2009 ɩ 22 ɩ 44 ɩ 66 ɩ ɩ ɩ ɩ 88 110 132 154 176 198 220

The Prostate Net Prostate Cancer Symposium Inaugural Meeting New York City October 6, 2009 Emerging Treatments on the Horizon Celestia S. Higano MD, FACP Professor Medicine and Urology University of Washington Member, Fred Hutchison Cancer Research Center

Emerging Treatment Protocols Eric Klein, MD

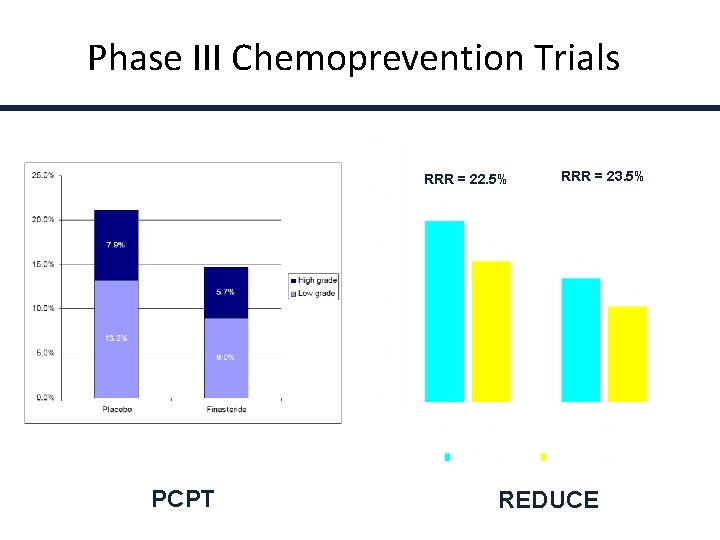
Phase III Chemoprevention Trials RRR = 22. 5% PCPT RRR = 23. 5% REDUCE

Prevention: What I Tell Patients • Low calorie diet • Exercise • Vitamin E and Selenium don’t work • Data on other dietary changes and supplements not proven • 5 -alpha reductase inhibitors are safe and effective, and the only intervention supported by results of Phase III controlled trials

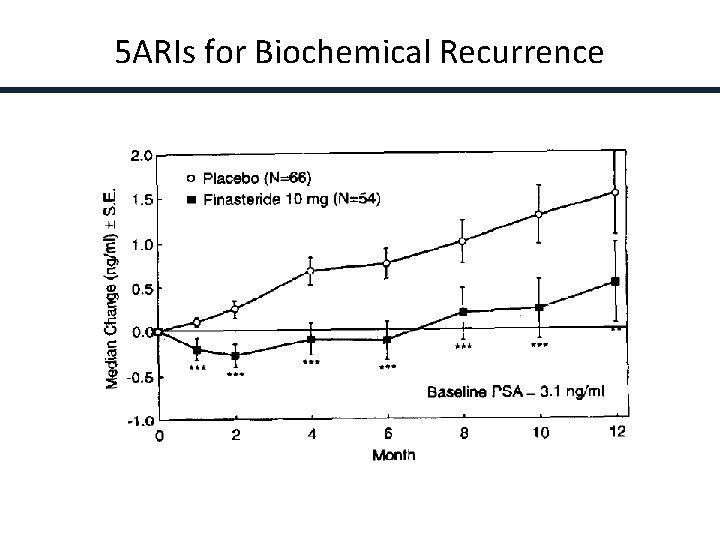
5 ARIs for Biochemical Recurrence Andriole et al, Urology 45: 491, 1995

ARTS Study Design Multicenter, randomized, double-blind, placebo-controlled trial (n=276) Randomization Baseline Negative bone scan PSA nadir Treatment period 2 years Follow-up Placebo Screening -3 0 weeks End of treatment Avodart (dutasteride) 0. 5 mg/day 3 6 9 12 15 Months 18 21 24 +4 months

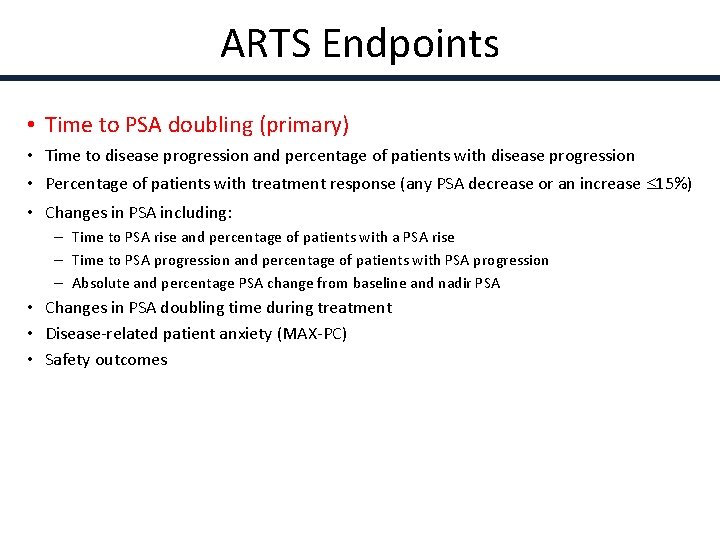
ARTS Endpoints • Time to PSA doubling (primary) • Time to disease progression and percentage of patients with disease progression • Percentage of patients with treatment response (any PSA decrease or an increase 15%) • Changes in PSA including: – Time to PSA rise and percentage of patients with a PSA rise – Time to PSA progression and percentage of patients with PSA progression – Absolute and percentage PSA change from baseline and nadir PSA • Changes in PSA doubling time during treatment • Disease-related patient anxiety (MAX-PC) • Safety outcomes
 Inaugural meeting agenda
Inaugural meeting agenda Mdv3100 prostate cancer
Mdv3100 prostate cancer Espérance de vie après récidive cancer prostate
Espérance de vie après récidive cancer prostate Stephen ko md
Stephen ko md The new prostate cancer infolink
The new prostate cancer infolink Prostate cancer staging
Prostate cancer staging Prostate cancer tnm classification
Prostate cancer tnm classification Aula inaugural de historia 6 ano
Aula inaugural de historia 6 ano Anastrophe example
Anastrophe example Identifying exponential growth and decay
Identifying exponential growth and decay Abraham lincoln vs jefferson davis venn diagram
Abraham lincoln vs jefferson davis venn diagram Jefferson davis inaugural address
Jefferson davis inaugural address Fdr political cartoons
Fdr political cartoons For todays meeting
For todays meeting What is meeting and types of meeting
What is meeting and types of meeting Meeting objective
Meeting objective Types of meeting
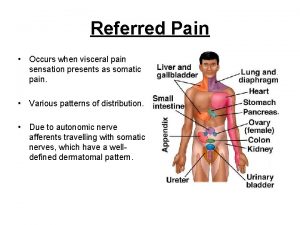
Types of meeting Viscerosomatic levels
Viscerosomatic levels Prostatic fluid
Prostatic fluid Prostate pathology
Prostate pathology Prostate referred pain
Prostate referred pain Celine duperron
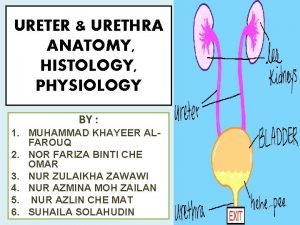
Celine duperron Ureter physiology
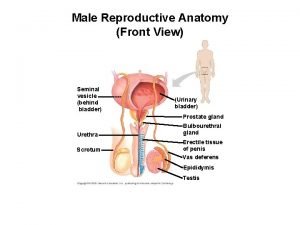
Ureter physiology Primary sex organ of the male reproductive system? *
Primary sex organ of the male reproductive system? * Eduard baco
Eduard baco Adenocarcinome prostate
Adenocarcinome prostate Prostate histology
Prostate histology Normal weight of prostate
Normal weight of prostate Prostate
Prostate Lipoma
Lipoma Spermatolysis
Spermatolysis Tuip prostate
Tuip prostate Prostate pathology
Prostate pathology Prostate
Prostate Verumontanum urethra
Verumontanum urethra Anatomie zonale de mac neal
Anatomie zonale de mac neal Function of prostate gland
Function of prostate gland Protocole irm prostate
Protocole irm prostate Dr zsigmond ildikó
Dr zsigmond ildikó Base of prostate gland
Base of prostate gland đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi