The Principles and Rationale of Patent Protection Ethical
















- Slides: 21

The Principles and Rationale of Patent Protection Ethical Implications of Patenting Academic Research University Foundation Brussels - 22 Nov. 2005 Alain Strowel Professor, Facultés Saint-Louis, Université de Liège and K. U. L. -K. U. B. , Avocat, Covington & Burling, Brussels astrowel@fusl. ac. be

Introduction: New Perception of Patent · Growing and increasingly controversial role of patents: – A critical topic for business, policy makers and interest groups, not only legal experts – The topic of increased critiques from NGOs, consumers groups, academics · Move from the realm of (patent) lawyers to the politic arena and in the press – For ex. F. T. , 22 Nov. 05: « Patently unfair? » about the patent war between branded drugs and generic companies • Pressure for affordable medicines: market response (bundling of branded and generic medicines)? · Long considered a technical area, patent law is now held up to public scrutiny 2

Table of Content · I. Principles of patent law – Comparison between patent, copyright and secrecy – Illustration: software · II. Rationale of patent protection – To spur innovation – Further economic benefits · III. Review of Issues – Extension of patentable inventions – Research in patent law 3

I. Principles of Patent Law (in Europe) At intergovernmental level: · 1973 European Patent Convention (EPC: 31 States): – Single application in Munich at the European Patent Office (EPO), but bundle of national patents – Centralized grant, but national enforcement · Draft European Patent Litigation Agreement (EPLA) At EU level: · Draft Community Patent Regulation - Single procedure (before the EPO) and EU-wide patent - Unitary judicial system · Harmonization Directives (divergences of existing law): - Biotech inventions Directive (1998) - Rejected: Computer-implemented inventions Directive (July 4 2005)

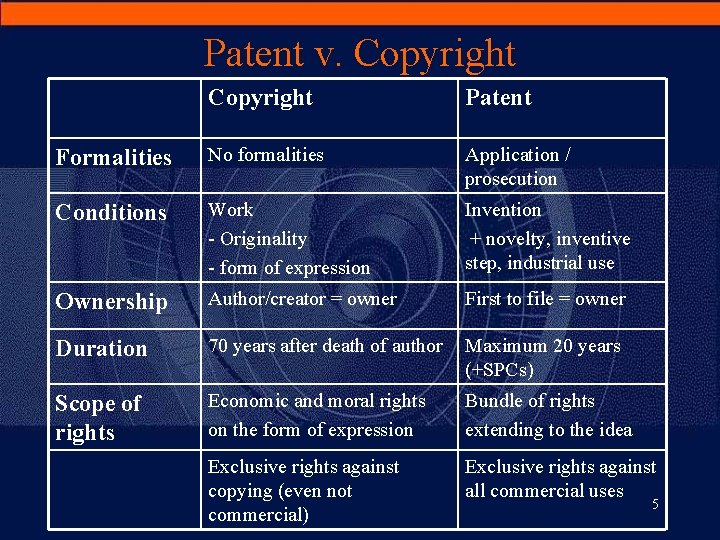
Patent v. Copyright Patent Formalities No formalities Application / prosecution Conditions Work - Originality - form of expression Invention + novelty, inventive step, industrial use Ownership Author/creator = owner First to file = owner Duration 70 years after death of author Maximum 20 years (+SPCs) Scope of rights Economic and moral rights on the form of expression Bundle of rights extending to the idea Exclusive rights against copying (even not commercial) Exclusive rights against all commercial uses 5

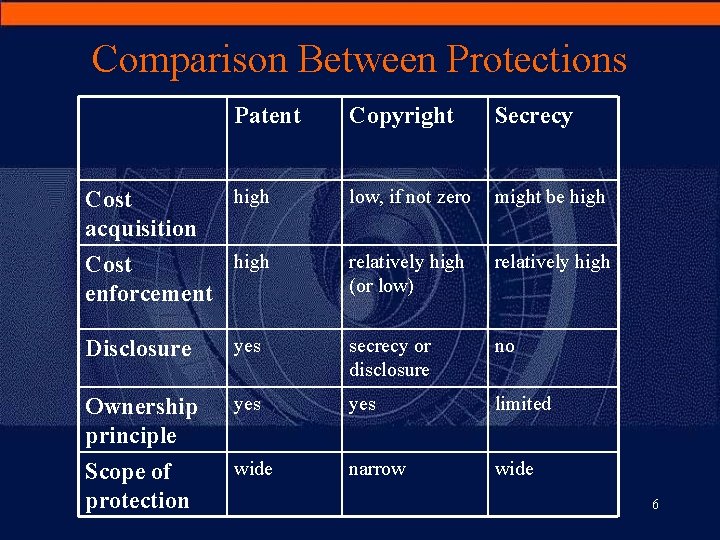
Comparison Between Protections Patent Copyright Secrecy high low, if not zero might be high Cost enforcement relatively high (or low) relatively high Cost acquisition Disclosure yes secrecy or disclosure no Ownership principle yes limited Scope of protection wide narrow wide 6

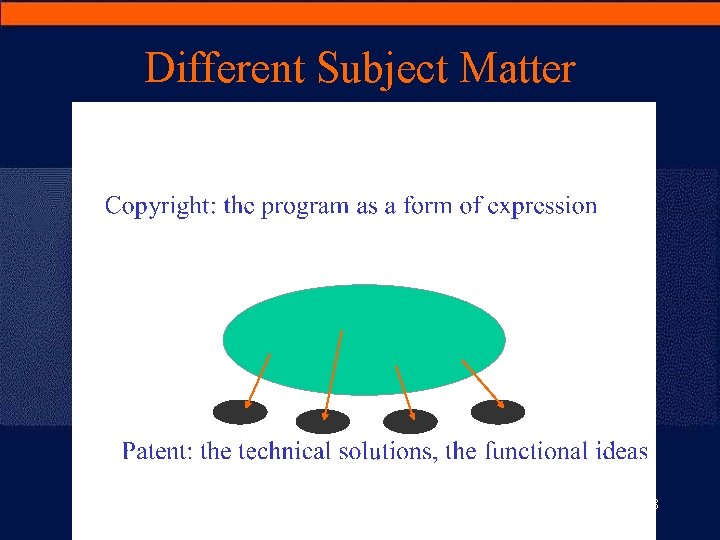
Copyright and Patent for Software · Software is code and has a literal aspect, but it also involves processes and has functional aspects – Similar dual protection of industrial design · Copyright – extends to the expression of a program in any form, including object and source code, but does not extend to ideas and principles which underlie any element of a computer program · Patent – covers a computer-implemented invention with a technical contribution, whether in source code or object code 7

Different Subject Matter 8

Different Objectives · EPO (IBM - T 1173/97): « The protections by copyright and patent constitute two different modes of protection which can nevertheless apply to the same object (for ex. a computer program) because each of them follows its own objective » · Objectives: – Copyright: to combat piracy (from users) – Patents: to ensure a market advantage (vis-à-vis competitors) 9

II. Patent Rationale To Spur Innovation · Patents promote innovation by allowing the inventor to recoup his investments – Objective: patent rights “should contribute to the promotion of technological innovation” (Art. 7 TRIPS) – But also “to the mutual advantage of producers and users of technological knowledge” (Art. 7 TRIPS) “The patent system added the fuel of interest to the fire of genius” Abraham Lincoln Patent permits the patentee “to derive the material benefit to which he is entitled as a reward for his intellectual effort and work, and compensation for the expenses which his research and experimentation leading to the invention have entailed. ” 10 WIPO Intellectual Property Handbook

Rationale for Patent Extension · 1992 Regulation on Supplementary Protection Certificates Recitals “Whereas medicinal products, especially those that are the result of long, costly research will not continue to be developed in the Community and in Europe unless they are covered by favorable rules that provide sufficient protection to encourage such research; Whereas at the moment the period that elapses between the filing of an application for a patent for a new medicinal product and authorization to place the medicinal product on the market makes the period of effective protection under the patent insufficient to cover the investment put into the research; ” 11

Rationale for Additional Protection · 2000 Regulation on Orphan Medicinal Products (market exclusivity for rare diseases) Recitals: “Some conditions occur so infrequently that the cost of developing and bringing to the market a medicinal product to diagnose, prevent or treat the condition would not be recovered by the expected sales of the medicinal product; the pharmaceutical industry would be unwilling to develop the medicinal product under normal market conditions; these medicinal products are called ‘orphan’; ” […] Experience in the United States of America and Japan shows that the strongest incentive for industry to invest in the development and marketing of orphan medicinal products is where there is a prospect of obtaining market exclusivity for a certain number of years during which part of the investment might be recovered; data protection under Article 4(8)(a)(iii) of Council Directive 65/65/EEC of 26 January 1965 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (1) is not a sufficient incentive for that purpose; ” 12

Further Economic Rationale for Patent · To initiate a dynamic cycle leading to economic growth ”The inventor’s reward is financial gain, and he is motivated to repeat the process again, investing some of his gain in new R&D for new inventions. This process becomes a dynamic cycle of change which generates changes in other areas. He is also likely to hire and train others, or transact business with others, who will in turn be motivated to invent and create products by the prospect of financial gain. Not only will the R&D lead to associated inventions by others, it is also likely to stimulate other economic consequences such as increased employment…” Intellectual Property, WIPO, 2003 13

Further Economic Rationale for Patent · To promote the financing of startups ”To a large extent … the best justification – and sometimes, to be truthful, the only one- for the system appears to be to promote the financing of dynamic new entrants. ” Prof. R. Merges 14

Further Economic Rationale for Patent · To manage the risks – The level of investment (in R&D for ex. ) is not enough to ensure economic benefits: quality of spending and effective management of innovation is as important as R&D investments · The patent system contributes to manage the risks related to the R&D investments: – Measure for R&D output – Basis for licensing 15

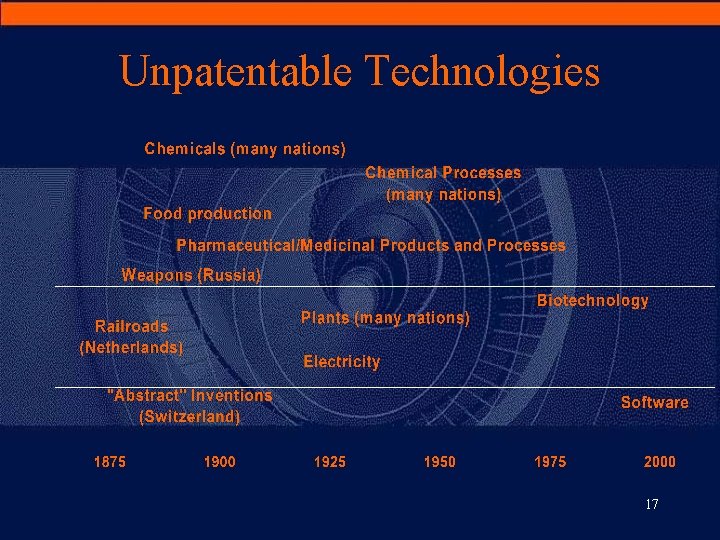
III. Selected Issues Extension of Patentable Inventions · • “The industry argued that the particular technological conditions in the field made inventions in the field unsuitable for patent protection. It is much more difficult than in the case of mechanical processes to draw the correct line of demarcation between what is and is not ‘invention. ’” Schiff’s summary of the 1883 Zurich Congress 16

Unpatentable Technologies 17

Dematerialization of Knowledge · Before: – technical knowledge is about material things (mechanics) · Now: – with biotech and IT patents, the knowledge is about intangibles • biotech patents could apply to genetic information which is more upstream from research than traditional inventions • IT patents could apply to the functionality of an algorithm · Nevertheless, patent exclusions in Europe (>< US) on abstract/non-technical subject matter (Art. 52 EPC) remain: – mathematical methods, scientific theories, rules for playing games – discoveries: limit for biotech inventions – business methods, software « as such » : limit for computer-implemented inventions 18

Research in Patent Law · Built-in limitations in favor of (downstream) research: – Exclusion of discoveries • But difficulty to delineate discoveries and inventions in the biotech sector – Experimental use exception in patent law + « Bolar » exception for clinical trials (pharmaceuticals) · Research tools = resources used to make discoveries – Contractual issues: some research tool patent holders seek royalties under their patent licences that reach through their patent rights (reach-through patents) and attach to discoveries made using the tools – Overlapping patent rights can lead to obstructions to innovation 19

Concluding Remarks · Need to fine-tune some patent rules – Incredible changes over the last decade in the pharma/biotech patents area (where stronger public policy issues) · Contract-related issues (patents thickets and royalty stacking) exist: – Issue = patent licencing, rather than patent rules · Trade-off within patent law (exclusive right v. publication) is preferable than secrecy 20

Thank you for your attention 21