The PPP Model Works Policy Implications Dr M




- Slides: 20

The PPP Model Works Policy Implications Dr M Moran m. moran@lse. ac. uk Pharmaceutical R&D Policy Project Wellcome Trust London School of Economics October 2005

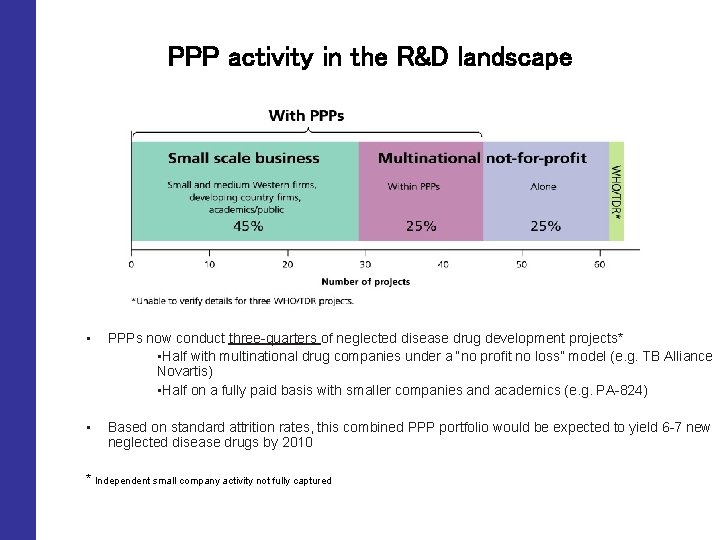
PPP activity in the R&D landscape • PPPs now conduct three-quarters of neglected disease drug development projects* • Half with multinational drug companies under a “no profit no loss” model (e. g. TB Alliance Novartis) • Half on a fully paid basis with smaller companies and academics (e. g. PA-824) • Based on standard attrition rates, this combined PPP portfolio would be expected to yield 6 -7 new neglected disease drugs by 2010 * Independent small company activity not fully captured

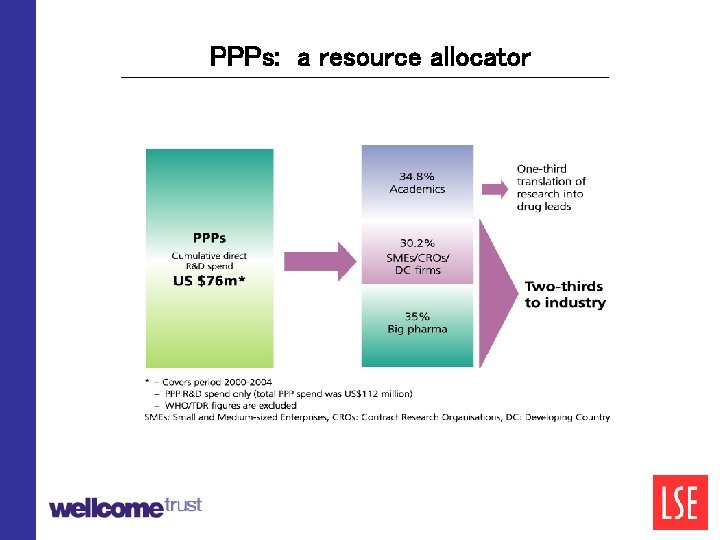
The role of PPPs • They integrate the development process across multiple partners and/or subcontractors • As PPPs mature, they function as a portfolio manager, selecting and de-selecting projects across the R&D spectrum from drug discovery to clinical trials • They act as a fund manager or resource allocator, channelling philanthropic and public funds to the “right” kind of projects from a public health perspective

PPPs: a resource allocator

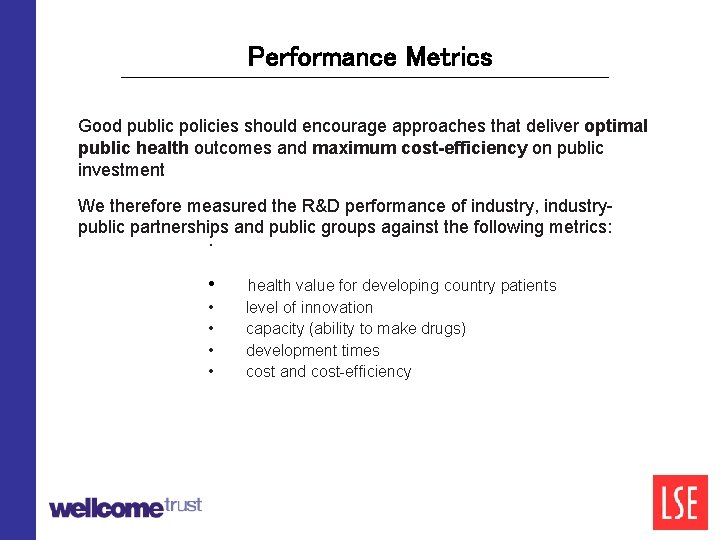
Performance Metrics Good public policies should encourage approaches that deliver optimal public health outcomes and maximum cost-efficiency on public investment We therefore measured the R&D performance of industry, industrypublic partnerships and public groups against the following metrics: : • • • health value for developing country patients level of innovation capacity (ability to make drugs) development times cost and cost-efficiency

PPPs deliver “higher health value” products than industry working alone Industry-alone • 12 of the 13 neglected disease products under the industry-alone model had a low overall health value to developing country patients e. g. rifapentin, rifabutin, PAS reformulation for TB Partnered • 3 of these 8 “partnered” products contributed significantly to reducing global health burdens • halved the global burden of onchocerciasis between 1990 and 2000 (ivermectin) • eradicated schistosomiasis in major parts of the world (praziquantel) • introduced the first suitable new paediatric anti-malarial for decades (Coartem)

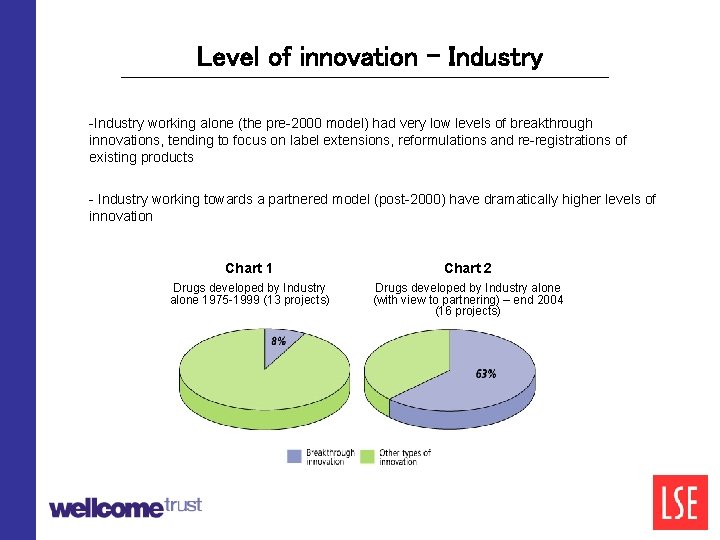
Level of innovation - Industry -Industry working alone (the pre-2000 model) had very low levels of breakthrough innovations, tending to focus on label extensions, reformulations and re-registrations of existing products - Industry working towards a partnered model (post-2000) have dramatically higher levels of innovation Chart 1 Chart 2 Drugs developed by Industry alone 1975 -1999 (13 projects) Drugs developed by Industry alone (with view to partnering) – end 2004 (16 projects)

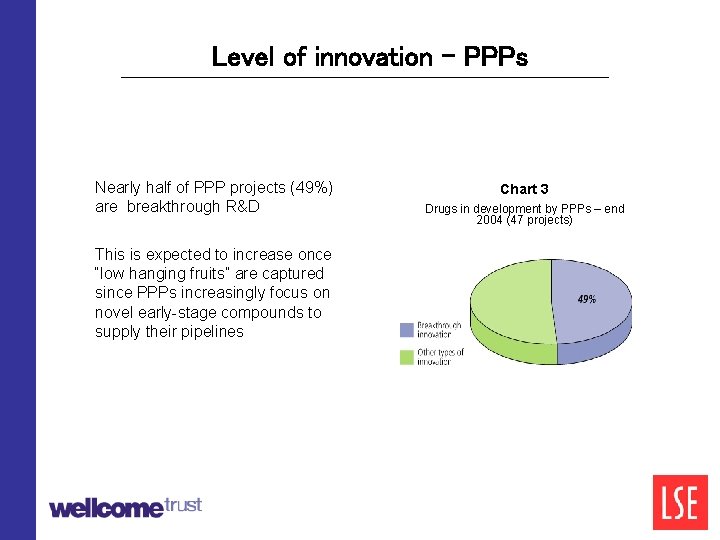
Level of innovation - PPPs Nearly half of PPP projects (49%) are breakthrough R&D This is expected to increase once “low hanging fruits” are captured since PPPs increasingly focus on novel early-stage compounds to supply their pipelines Chart 3 Drugs in development by PPPs – end 2004 (47 projects)

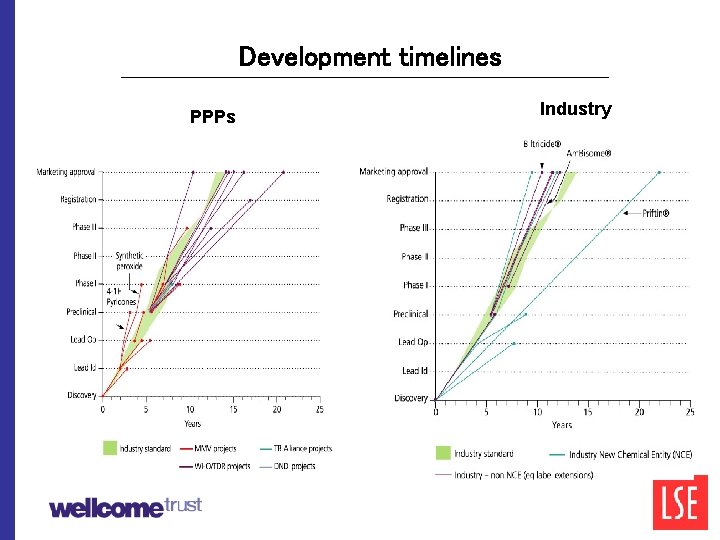
Development timelines PPPs Industry

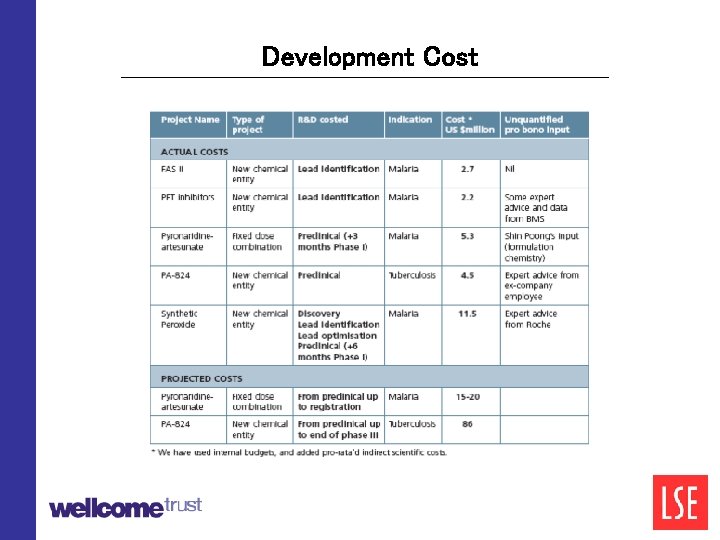
Development Cost

Correlates of R&D Success § A focus on neglected disease drug development for developing countries over all other considerations § Industry involvement from an early stage § Public involvement from an early stage § Appropriate use of the respective skills of the public and industry partners § Management and scientific staff with industrial drug-making experience § Adequate funding § Larger portfolios PPPs most closely match this framework (but not entirely!) 0%

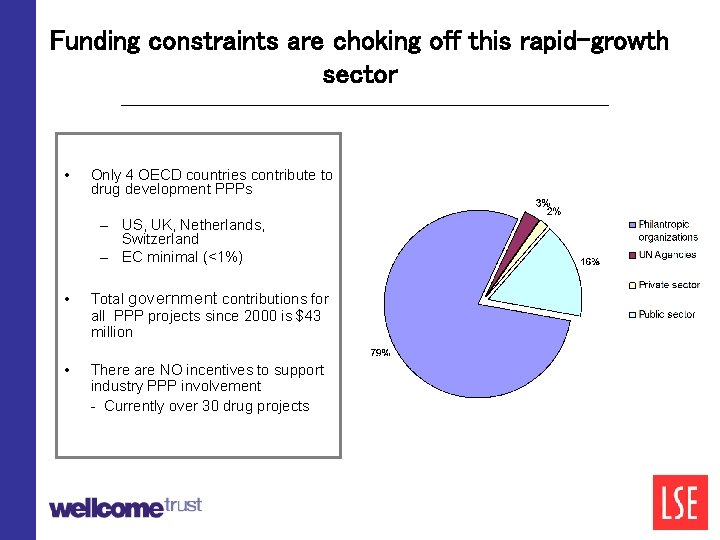
Funding constraints are choking off this rapid-growth sector • Only 4 OECD countries contribute to drug development PPPs – US, UK, Netherlands, Switzerland – EC minimal (<1%) • Total government contributions for all PPP projects since 2000 is $43 million • There are NO incentives to support industry PPP involvement - Currently over 30 drug projects

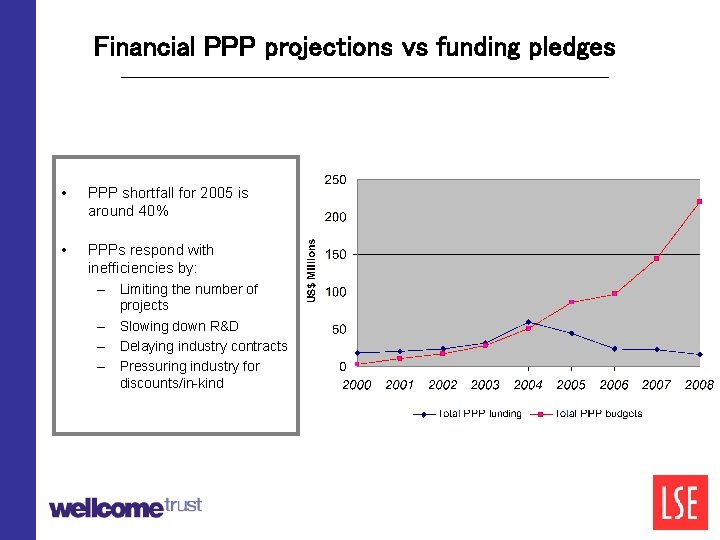
Financial PPP projections vs funding pledges • PPP shortfall for 2005 is around 40% • PPPs respond with inefficiencies by: – Limiting the number of – – – projects Slowing down R&D Delaying industry contracts Pressuring industry for discounts/in-kind

PRPP Proposals Based on these findings, we have developed several policy recommendations Our key recommendation is for an Industry R&D Facilitation Fund (IRFF) to support industry involvement in PPPs and improve the efficiency of PPP performance

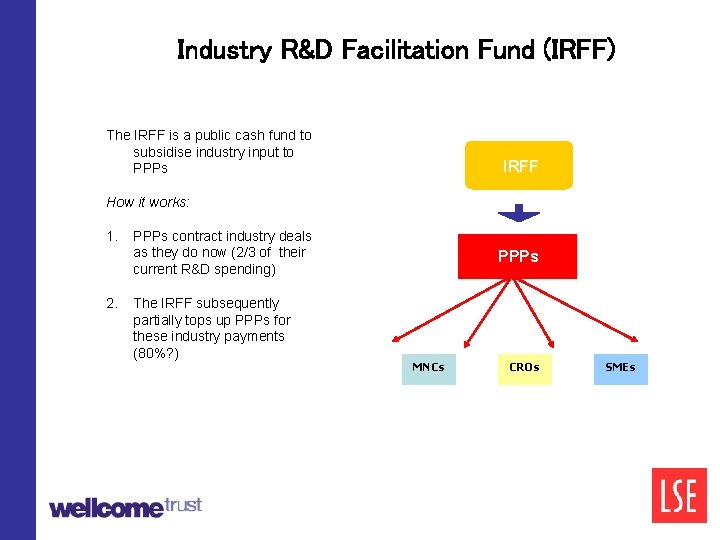
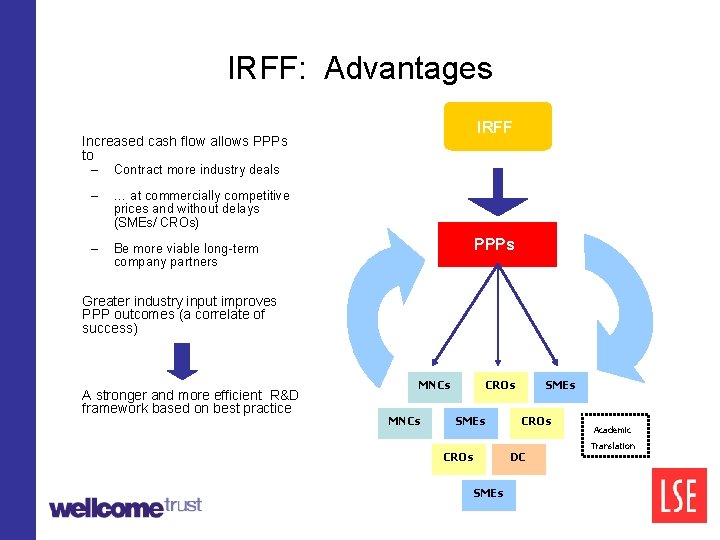
Industry R&D Facilitation Fund (IRFF) The IRFF is a public cash fund to subsidise industry input to PPPs IRFF How it works: 1. PPPs contract industry deals as they do now (2/3 of their current R&D spending) 2. The IRFF subsequently partially tops up PPPs for these industry payments (80%? ) PPPs MNCs CROs SMEs

IRFF: Advantages IRFF Increased cash flow allows PPPs to – Contract more industry deals – … at commercially competitive prices and without delays (SMEs/ CROs) – Be more viable long-term company partners IRFF PPPs Greater industry input improves PPP outcomes (a correlate of success) A stronger and more efficient R&D framework based on best practice MNCs CROs SMEs CROs DC SMEs Academic Translation

IRFF: advantages • Improved efficiency of funding: ØThe best performers are the highest users ØFunds are allocated in exactly the right amount at the right time across all neglected disease PPP projects (>40 projects) • Public risk and “pick the winner” are reduced: ØIndustry/health experts in PPPs select projects and partners rather than governments ØRisk is spread across total ND portfolio • Minimum new infrastructure is needed (VC host? ) • Highly cost-effective (efficient model; efficient funding mechanism)

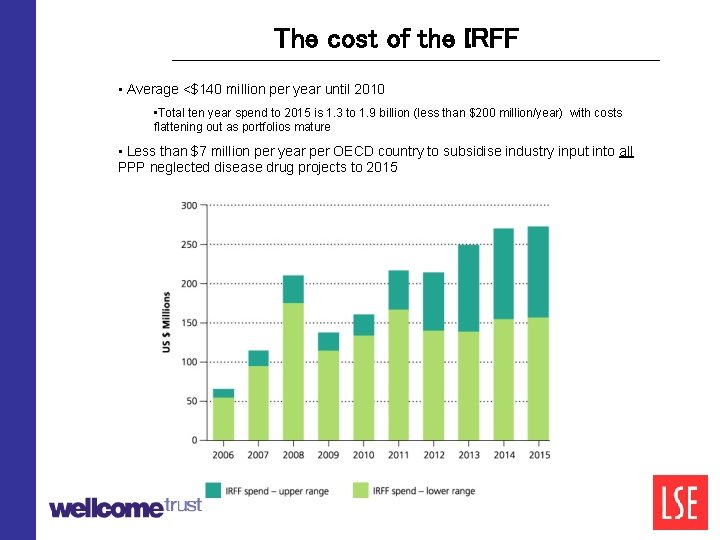
The cost of the IRFF • Average <$140 million per year until 2010 • Total ten year spend to 2015 is 1. 3 to 1. 9 billion (less than $200 million/year) with costs flattening out as portfolios mature • Less than $7 million per year per OECD country to subsidise industry input into all PPP neglected disease drug projects to 2015

Conclusions Governments should preferentially fund industry-public partnered approaches if they want to maximise health impact and value-for-money The IRFF offers governments a simple, effective mechanism to achieve these goals

0%