The Polarizing Microscope The electromagnetic spectrum visible light
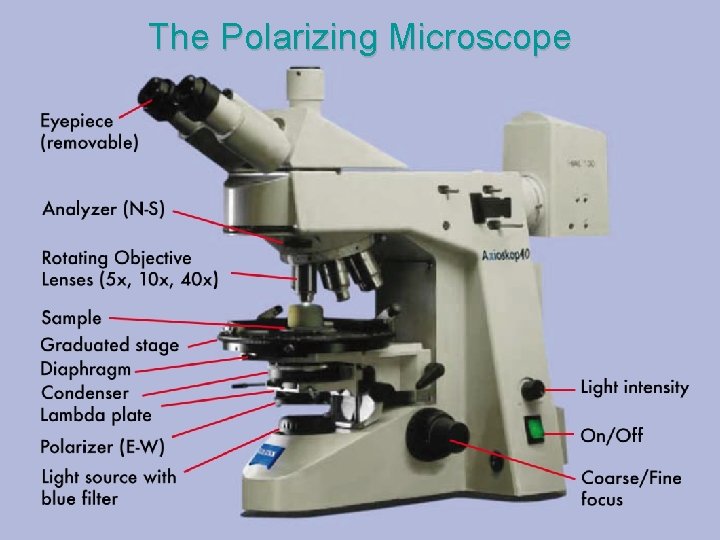
The Polarizing Microscope
The electromagnetic spectrum visible light (l = wavelength) Nesse, 2004; Fig. 6. 6
The electromagnetic spectrum visible light (l = wavelength) polychromatic light (white) full spectrum of visible l Nesse, 2004; Fig. 6. 6
The electromagnetic spectrum visible light (l = wavelength) polychromatic light (white) full spectrum of visible l monochromatic light – single l Nesse, 2004; Fig. 6. 6
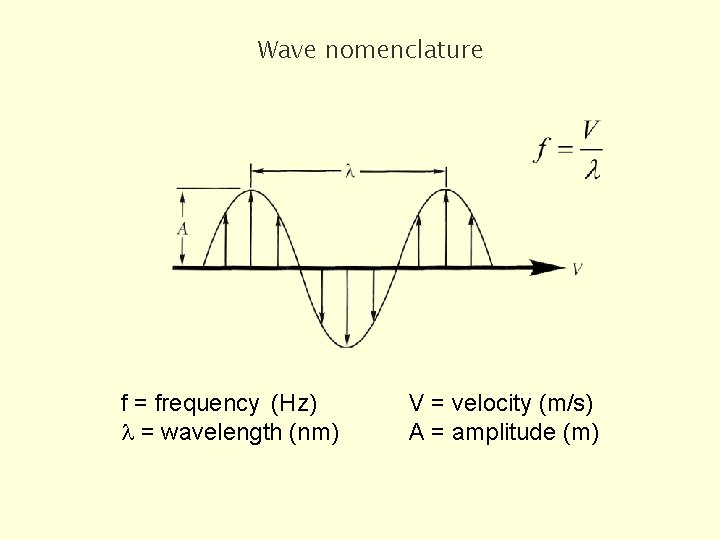
Wave nomenclature f = frequency (Hz) l = wavelength (nm) V = velocity (m/s) A = amplitude (m)
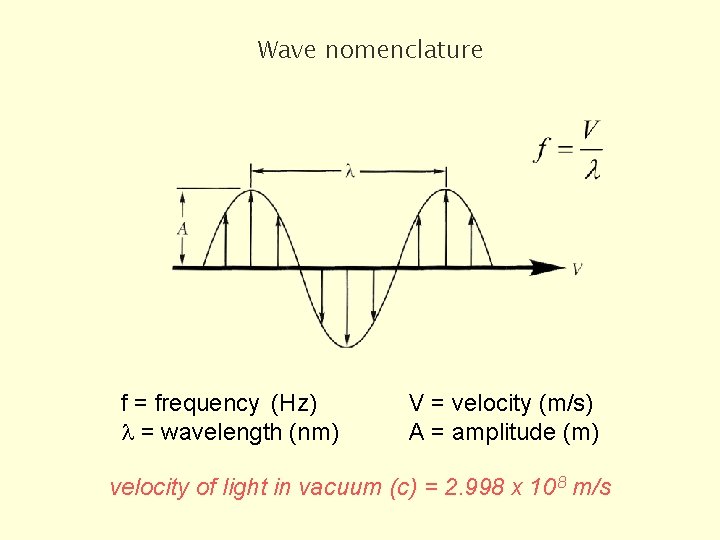
Wave nomenclature f = frequency (Hz) l = wavelength (nm) V = velocity (m/s) A = amplitude (m) velocity of light in vacuum (c) = 2. 998 x 108 m/s
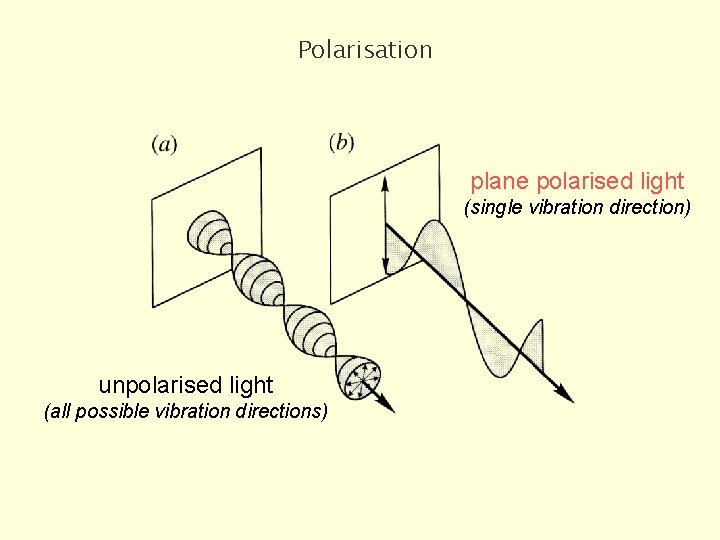
Polarisation plane polarised light (single vibration direction) unpolarised light (all possible vibration directions)
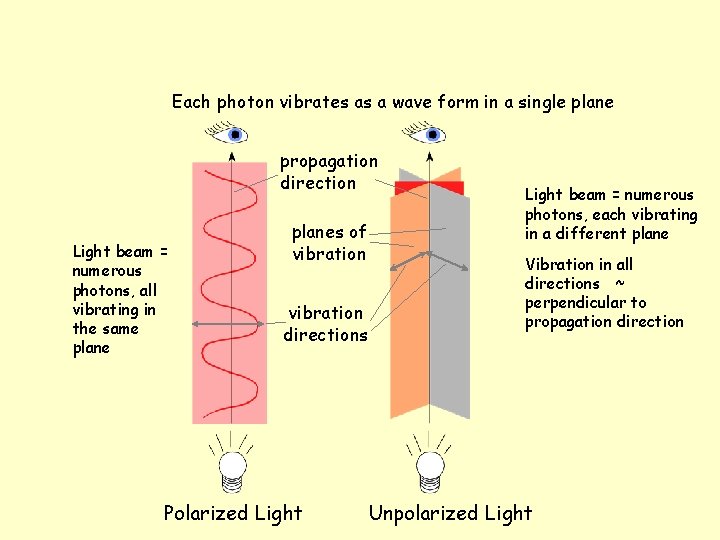
Each photon vibrates as a wave form in a single plane propagation direction Light beam = numerous photons, all vibrating in the same planes of vibration directions Polarized Light beam = numerous photons, each vibrating in a different plane Vibration in all directions ~ perpendicular to propagation direction Unpolarized Light
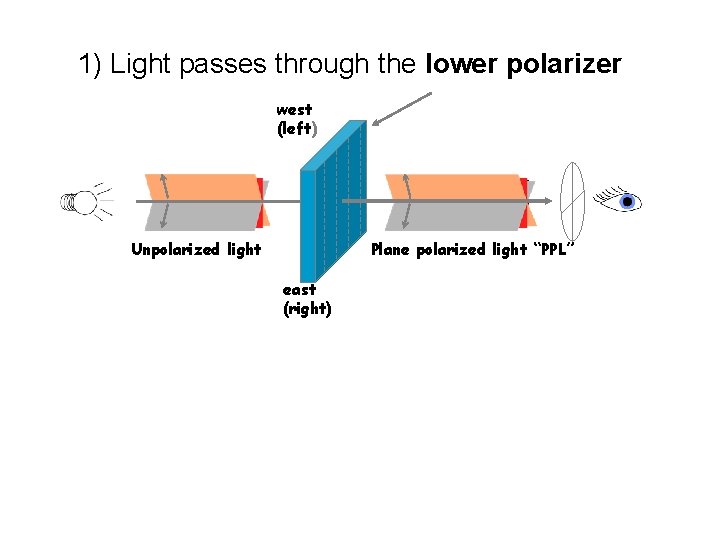
1) Light passes through the lower polarizer west (left) Unpolarized light Plane polarized light “PPL” east (right)
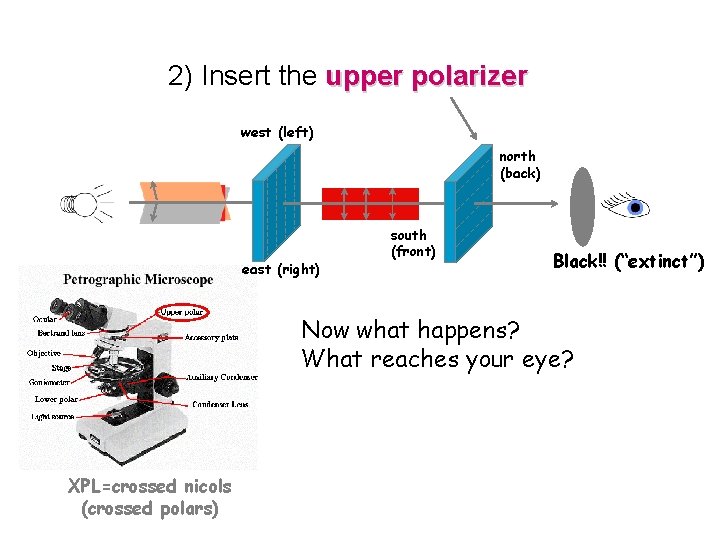
2) Insert the upper polarizer west (left) north (back) east (right) south (front) Black!! (“extinct”) Now what happens? What reaches your eye? XPL=crossed nicols (crossed polars)
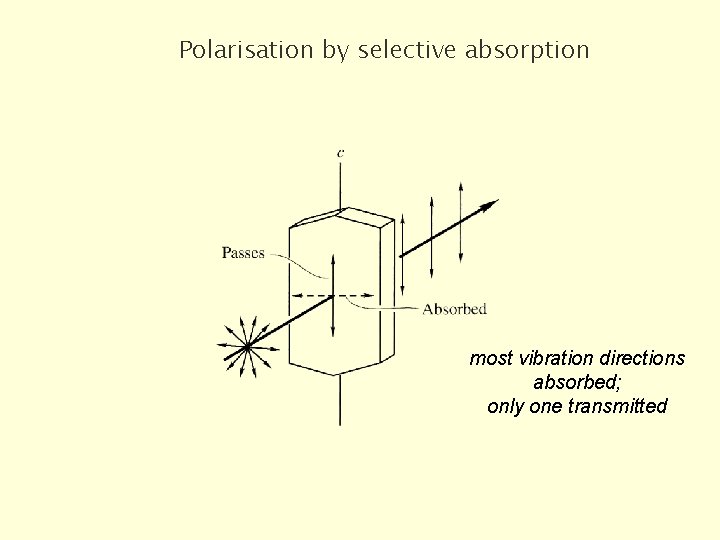
Polarisation by selective absorption most vibration directions absorbed; only one transmitted
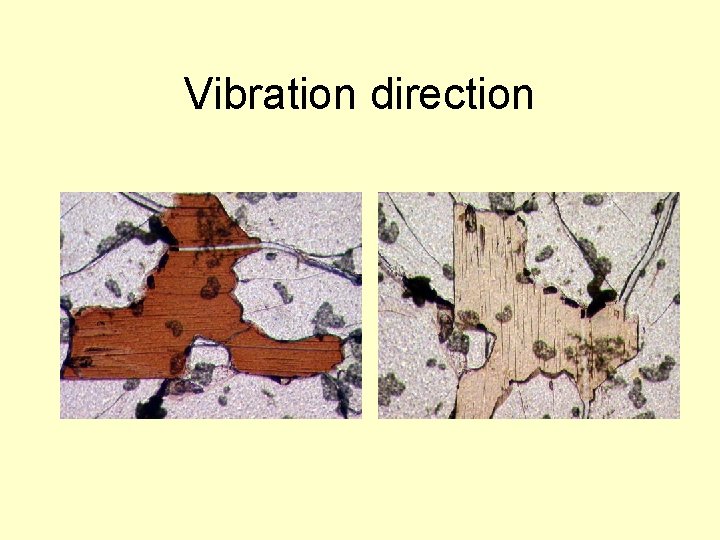
Vibration direction
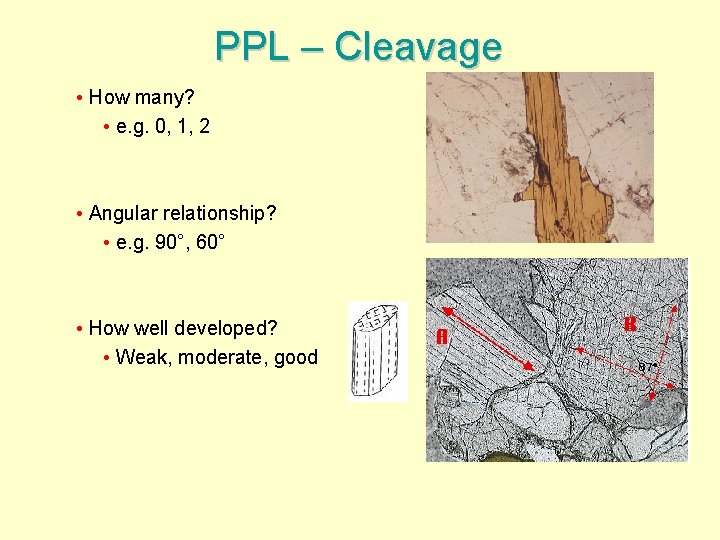
PPL – Cleavage • How many? • e. g. 0, 1, 2 • Angular relationship? • e. g. 90°, 60° • How well developed? • Weak, moderate, good
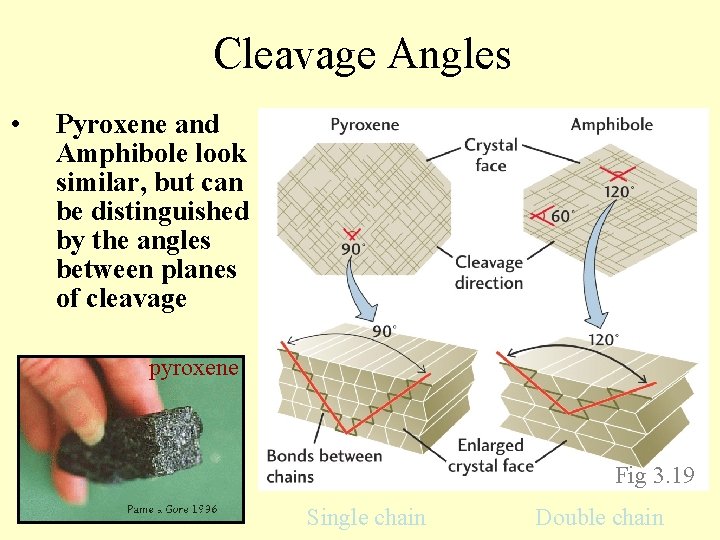
Cleavage Angles • Pyroxene and Amphibole look similar, but can be distinguished by the angles between planes of cleavage pyroxene Fig 3. 19 Single chain Double chain

Cleavage – number and orientation of cleavage planes Most easily observed in PPL (upper polarizer out), but visible in XN as well • No cleavages: quartz, olivine • 1 good cleavage: micas • 2 good cleavages: pyroxenes, amphiboles
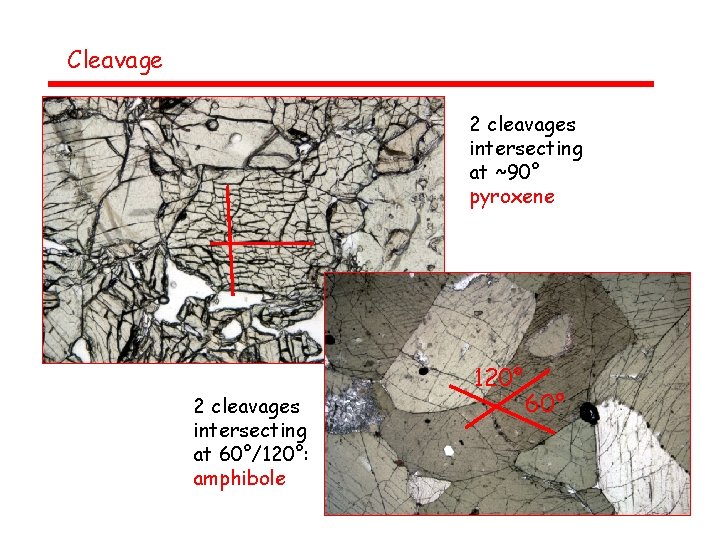
Cleavage 2 cleavages intersecting at ~90° pyroxene 2 cleavages intersecting at 60°/120°: amphibole 120° 60°
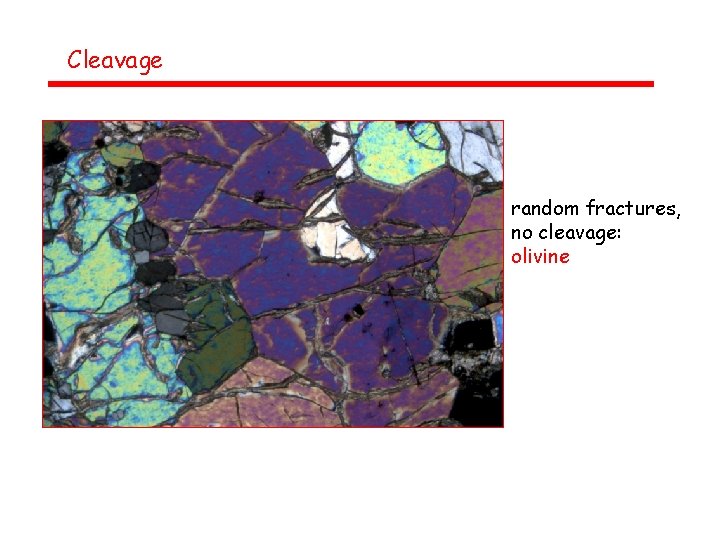
Cleavage random fractures, no cleavage: olivine
- Slides: 17