The phenol formaldehyde rxn Network formation Further reaction



- Slides: 24

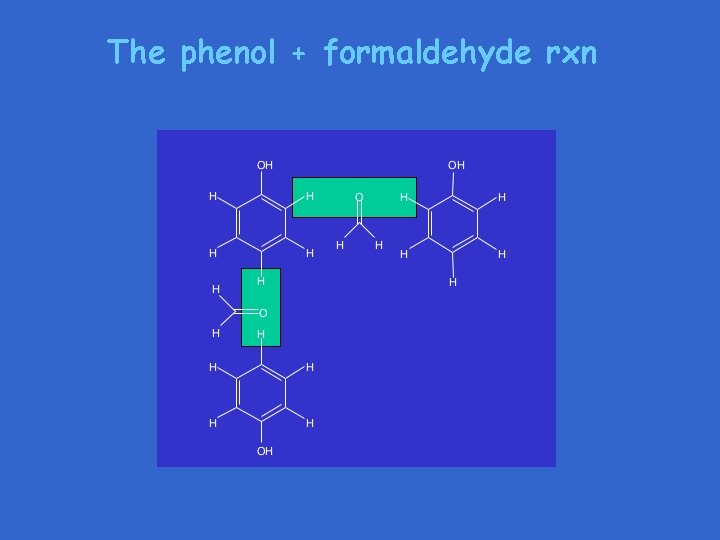
The phenol + formaldehyde rxn

Network formation Further reaction under heat & pressure builds up densely cross-linked network. This is Bakelite, a thermosetting polymer. Once reaction is complete, material cannot be reheated and/or reformed Bakelite

Bakelite - Material of a Thousand Uses Clear Bakelite items Bakelite camera Phenolic resin/celluloid clock Bakelite telephone Bakelite radio Bakelite microphone

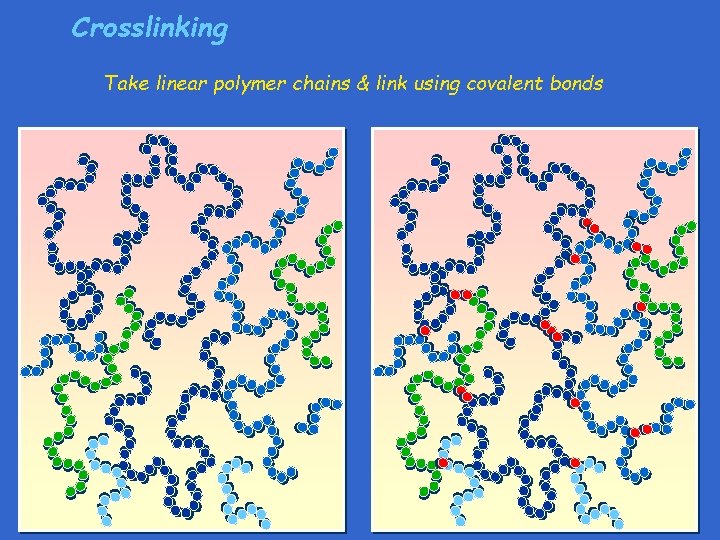
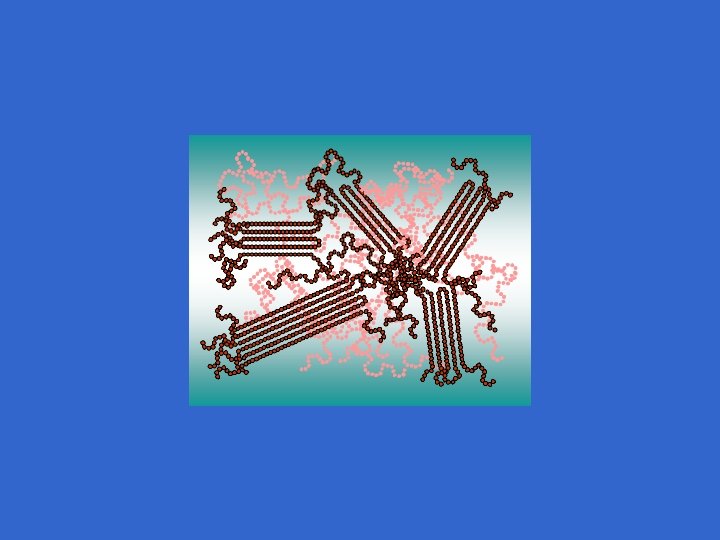
Crosslinking Take linear polymer chains & link using covalent bonds

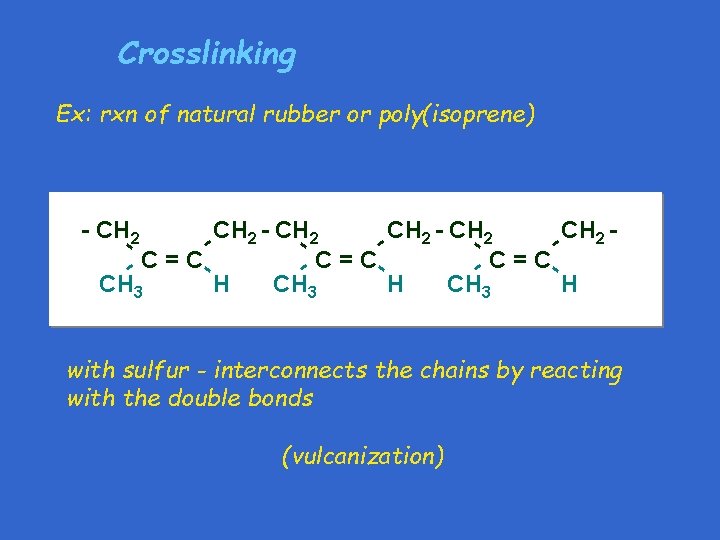
Crosslinking Ex: rxn of natural rubber or poly(isoprene) CH - CH 2 CH - 2 - 2 -C=C CH 3 H - - - CH 2 - - with sulfur - interconnects the chains by reacting with the double bonds (vulcanization)

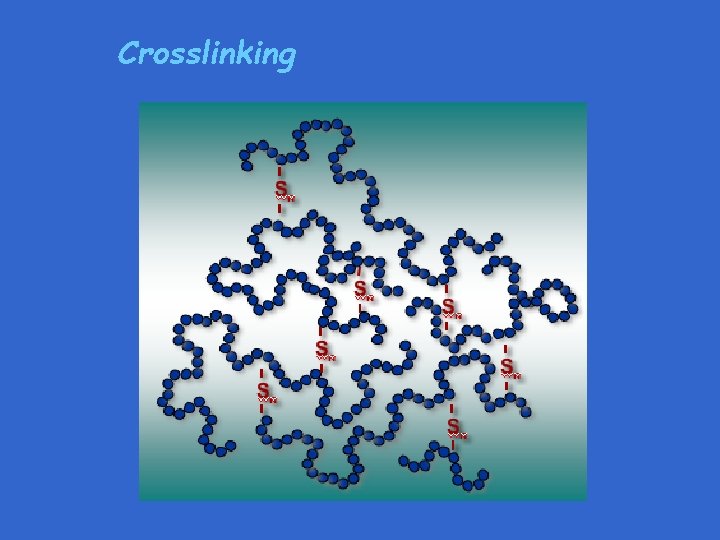
Crosslinking

Tacticity 3 types isotactic syndiotactic atactic

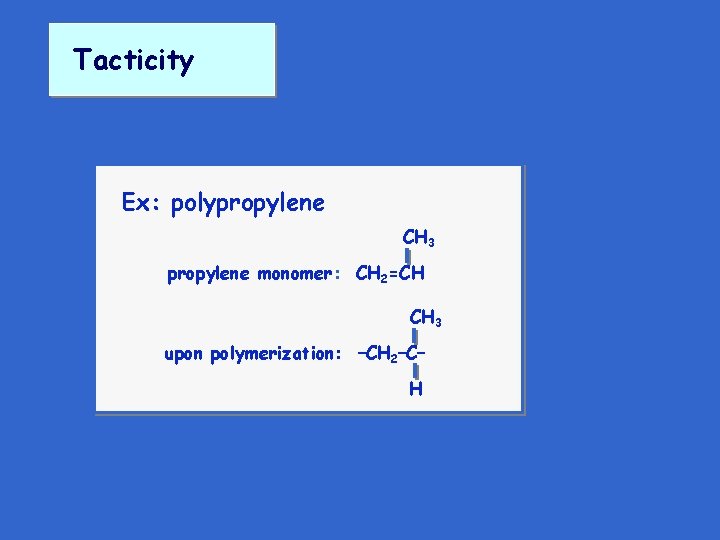
Tacticity Ex: polypropylene CH 3 propylene monomer: CH 2=CH CH 3 upon polymerization: –CH 2–C– H

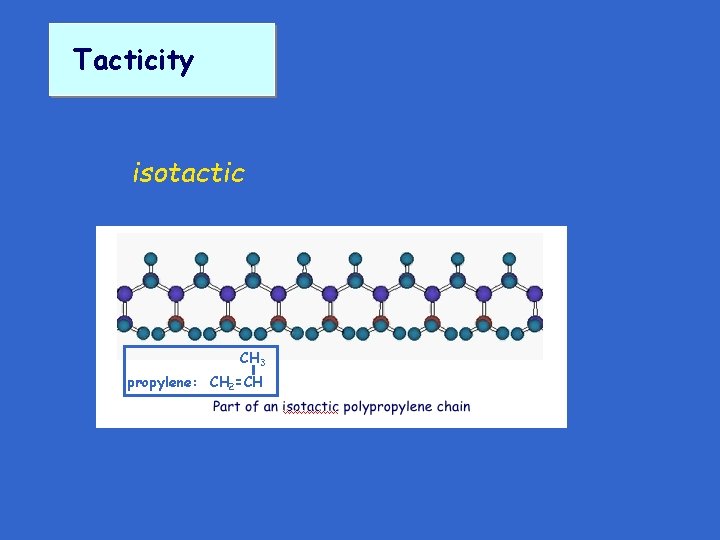
Tacticity isotactic CH 3 propylene: CH 2=CH

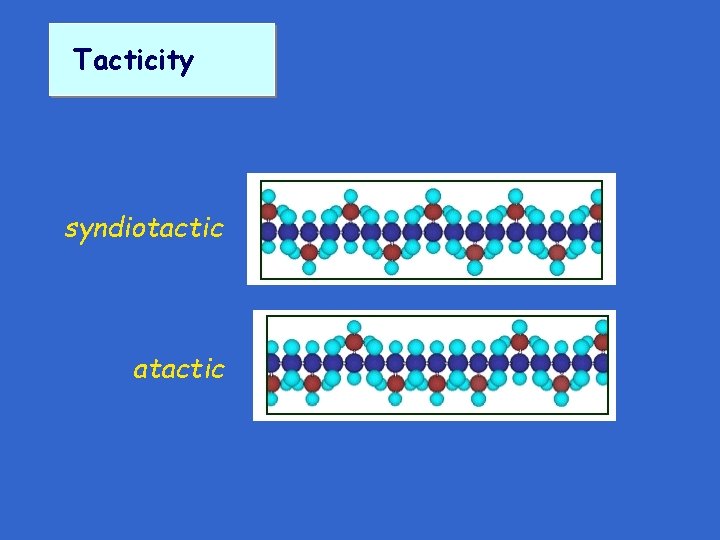
Tacticity syndiotactic atactic

Tacticity Polypropylene - largely isotactic character of PP allows "crystallization" as a result, material can be stiffer

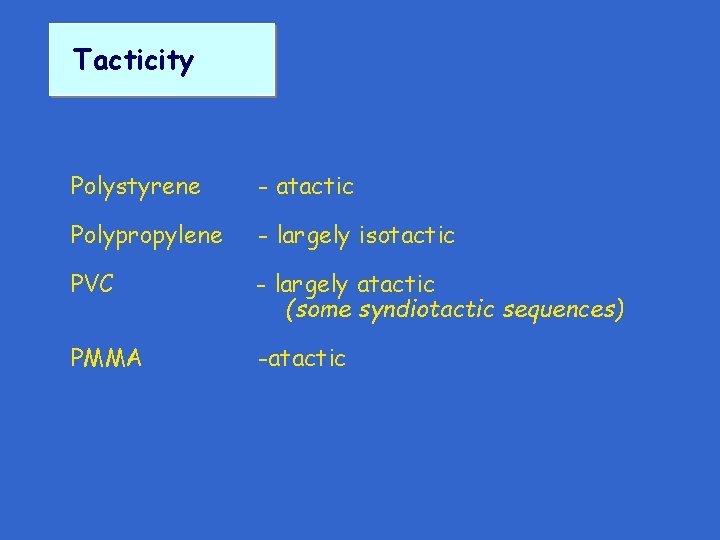
Tacticity Polystyrene - atactic Polypropylene - largely isotactic PVC - largely atactic (some syndiotactic sequences) PMMA -atactic

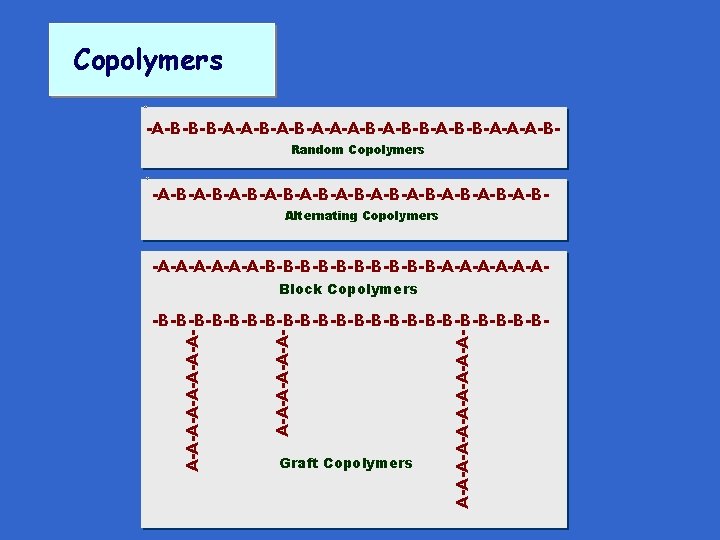
Copolymers -A-B-B-B-A-A-B-A-A-A-B-B-A-B-B-A-A-A-BRandom Copolymers -A-B-A-B-A-B-A-B-A-B-A-BAlternating Copolymers -A-A-A-B-B-B-B-B-A-A-ABlock Copolymers Graft Copolymers A-A-A-A-A-A-A-A- -B-B-B-B-B-B-B-B-B-B-B-

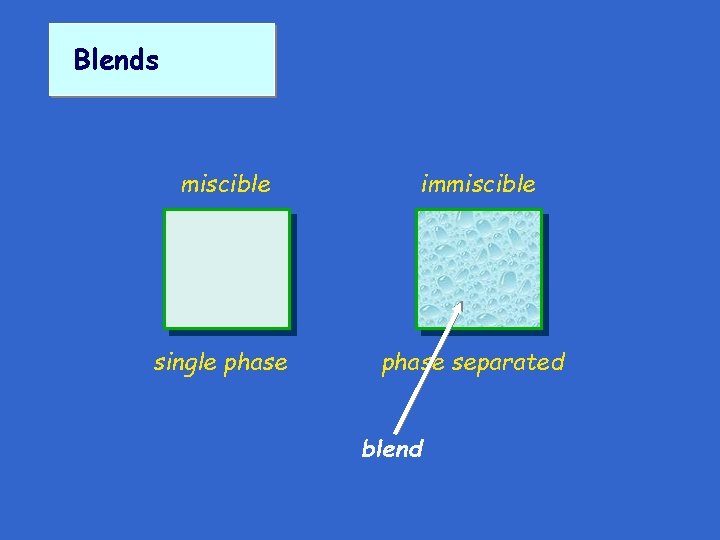
Blends miscible immiscible single phase separated blend

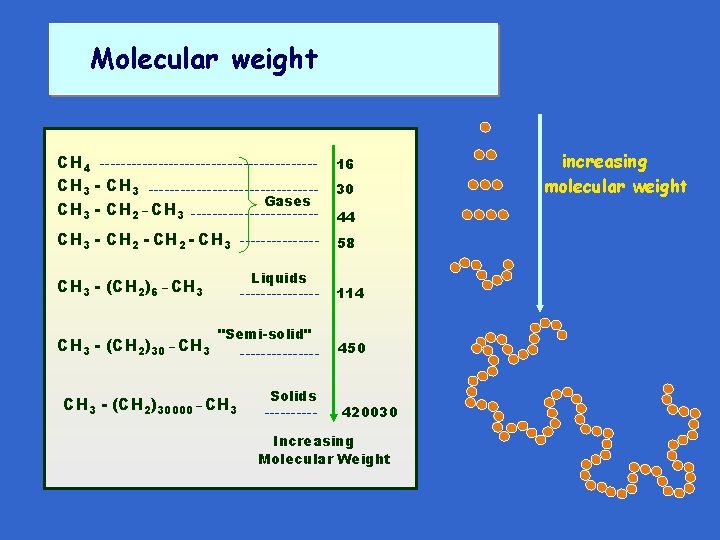
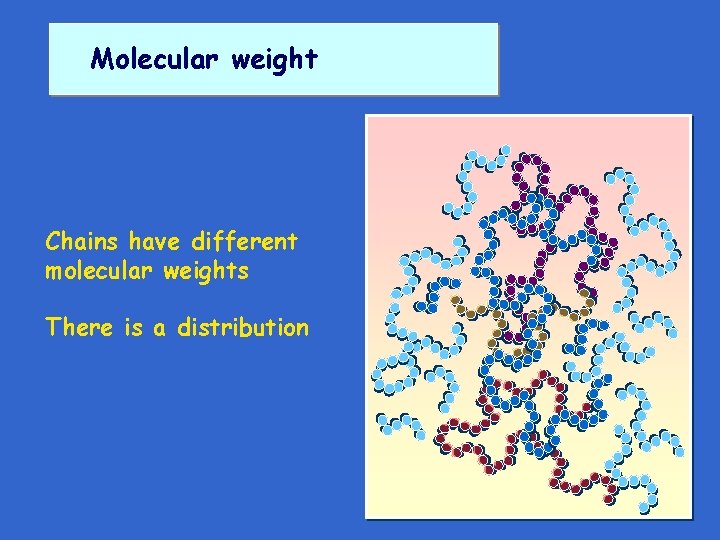
Molecular weight CH 4 --------------------CH 3 ----------------Gases CH 3 - CH 2 _ CH 3 ------------ 16 CH 3 - CH 2 - CH 3 -------- 58 Liquids CH 3 - (CH 2)6 _ CH 3 - (CH 2)30 _ CH 3 -------"Semi-solid" CH 3 - (CH 2)30000 _ CH 3 -------Solids ----- 30 44 114 450 420030 Increasing Molecular Weight increasing molecular weight

Molecular weight Chains have different molecular weights There is a distribution

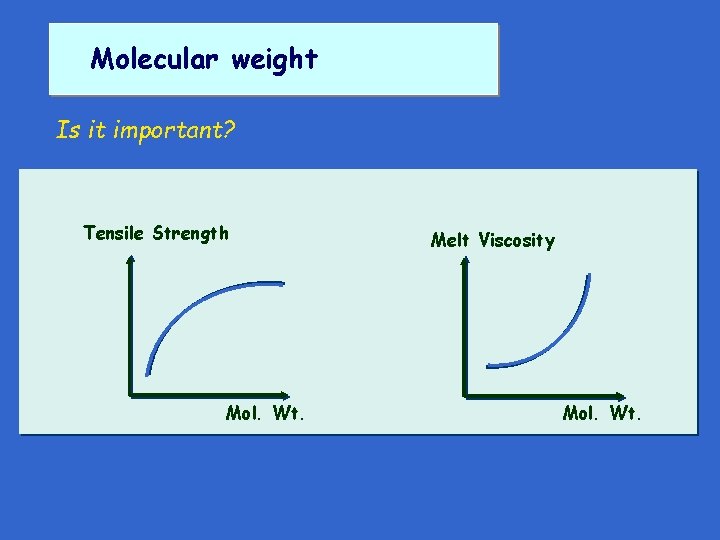
Molecular weight Is it important? Tensile Strength Mol. Wt. Melt Viscosity Mol. Wt.

Molecular weight Is it important? Very important in processing If viscosity too high, polymer difficult to process If too low, an extruded material won't hold together until it solidifies

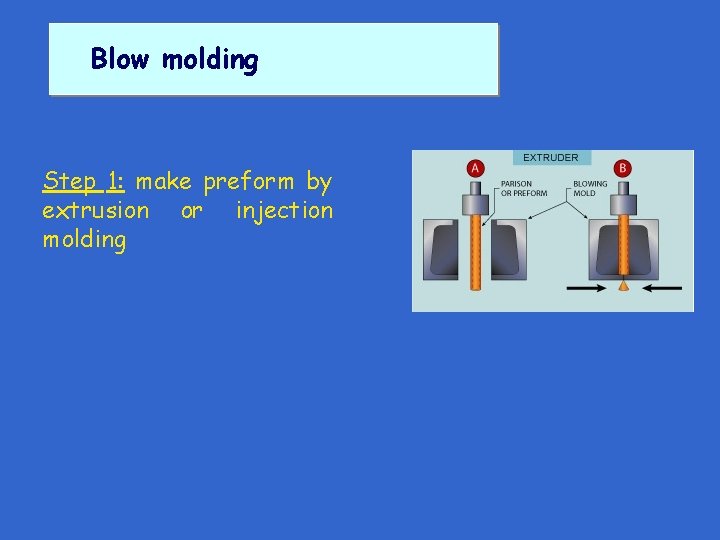
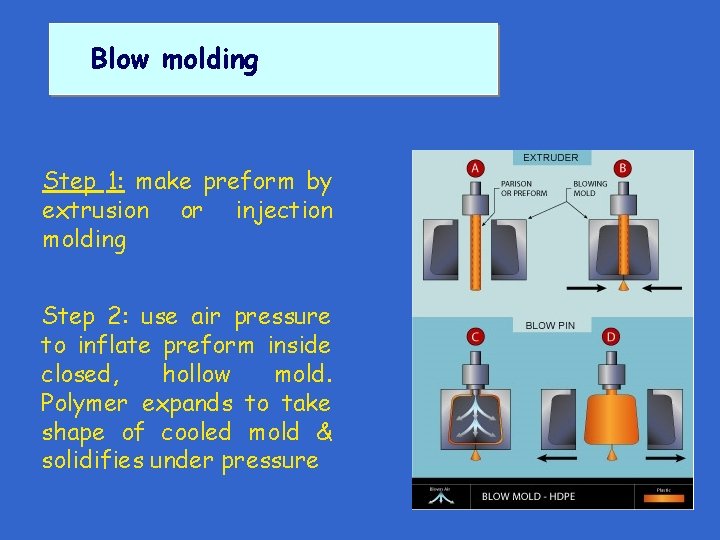
Blow molding Step 1: make preform by extrusion or injection molding

Blow molding Step 1: make preform by extrusion or injection molding Step 2: use air pressure to inflate preform inside closed, hollow mold. Polymer expands to take shape of cooled mold & solidifies under pressure

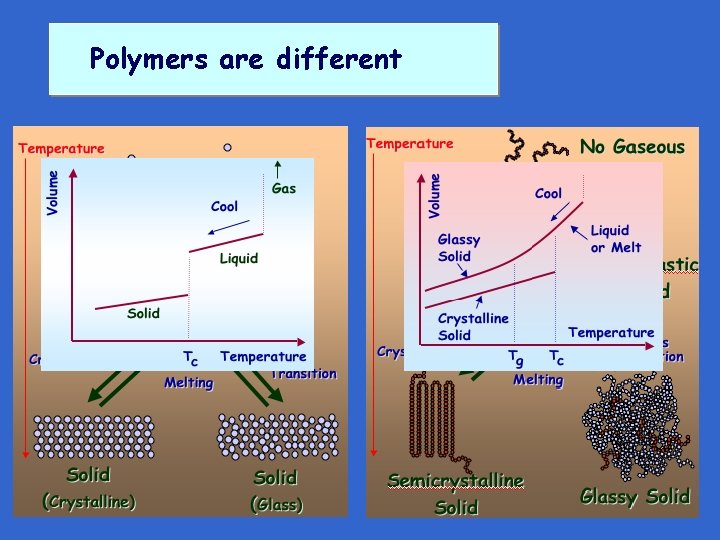
Polymers are different

Polymers are different • Normal crystalline materials – Either crystalline (~100 %, neglecting defects ) or amorphous at a particular temperature – Melt at a sharp, well-defined temperature • Crystallizable polymers – Never 100% crystalline – Melt over a range of temperatures

