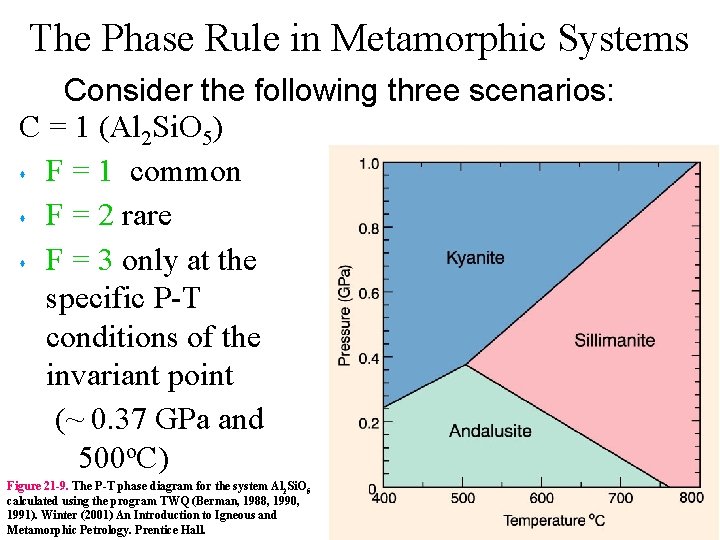
The Phase Rule in Metamorphic Systems Consider the

![Phyllosilicates Yellow = (OH) Serpentine: Mg 3 [Si 2 O 5] (OH)4 T-layers and Phyllosilicates Yellow = (OH) Serpentine: Mg 3 [Si 2 O 5] (OH)4 T-layers and](https://slidetodoc.com/presentation_image_h2/8678cff53cc1f8f2d97ea108bc339e25/image-12.jpg)

![Garnets Garnet: A 2+3 B 3+2 [Si. O 4]3 “Pyralspites” - B = Al Garnets Garnet: A 2+3 B 3+2 [Si. O 4]3 “Pyralspites” - B = Al](https://slidetodoc.com/presentation_image_h2/8678cff53cc1f8f2d97ea108bc339e25/image-20.jpg)




- Slides: 29

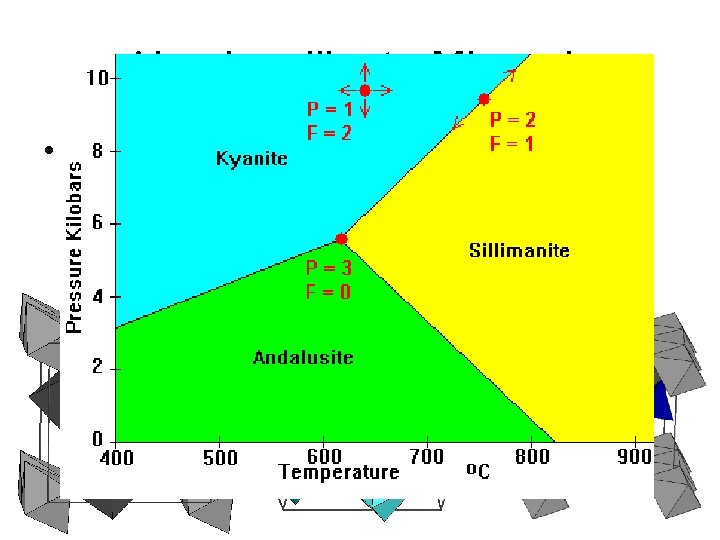
The Phase Rule in Metamorphic Systems Consider the following three scenarios: C = 1 (Al 2 Si. O 5) s F = 1 common s F = 2 rare s F = 3 only at the specific P-T conditions of the invariant point (~ 0. 37 GPa and 500 o. C) Figure 21 -9. The P-T phase diagram for the system Al 2 Si. O 5 calculated using the program TWQ (Berman, 1988, 1990, 1991). Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.

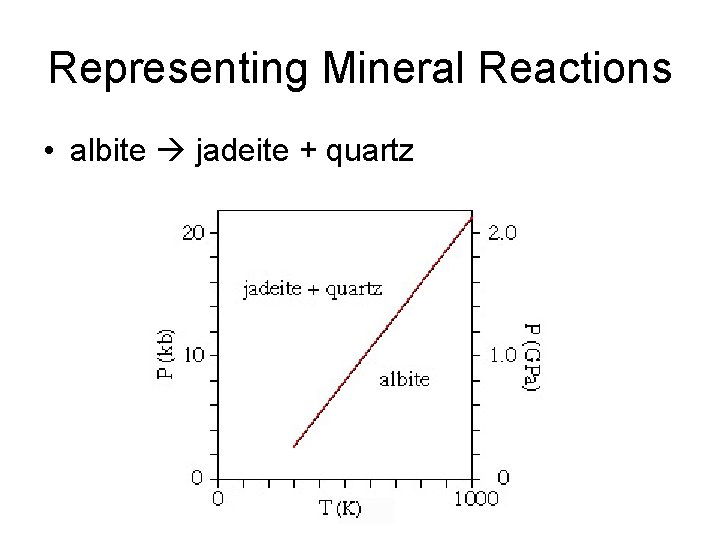
Representing Mineral Reactions • albite jadeite + quartz

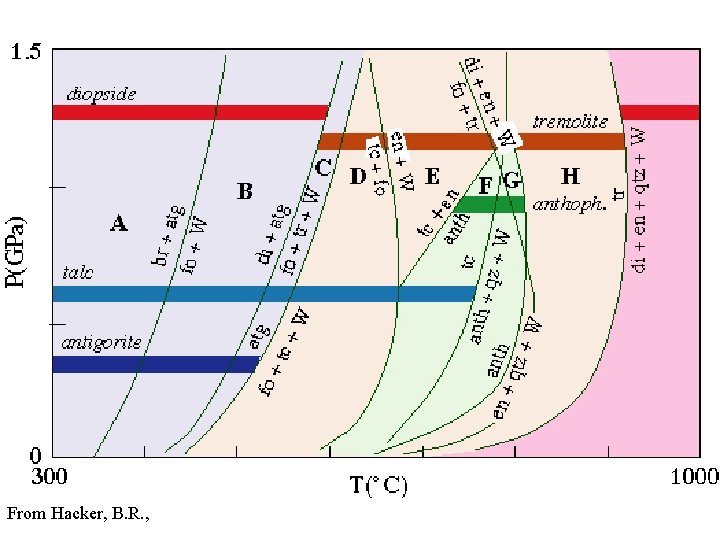
From Hacker, B. R. ,

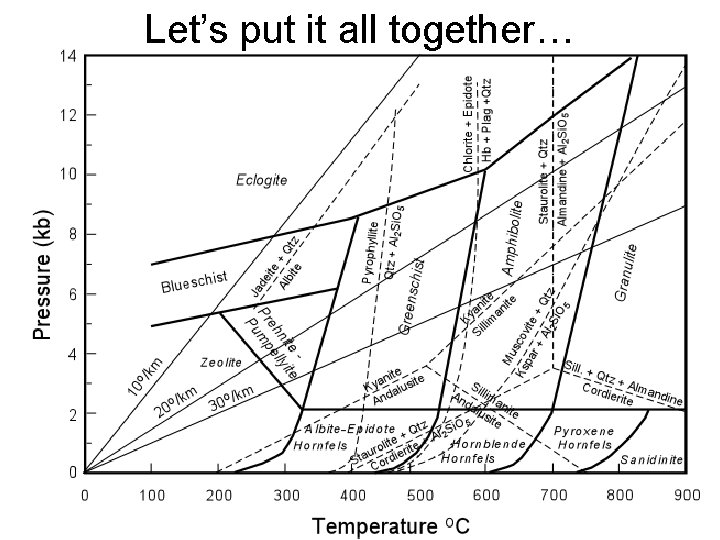
Let’s put it all together…

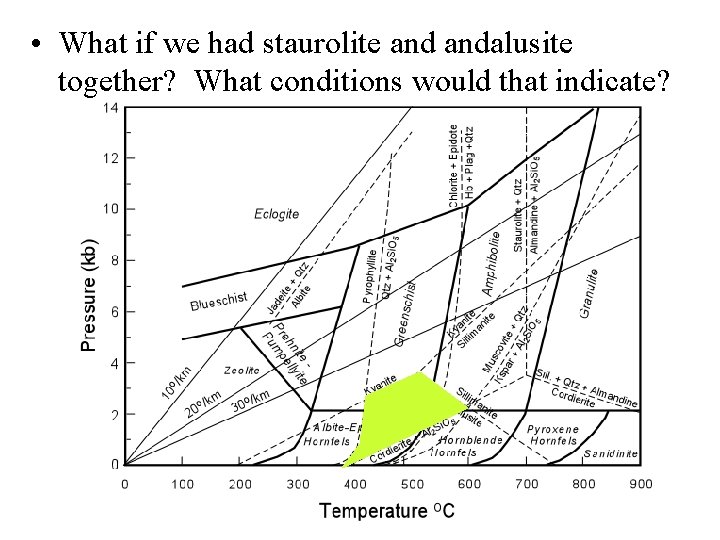
• What if we had staurolite andalusite together? What conditions would that indicate?

Metamorphic facies • P-T conditions, presence of fluids induces different metamorphic mineral assemblages (governed by thermodynamics/ kinetics) • These assemblages are lumped into metamorphic facies (or grades)


Aluminosilicate Minerals • SILLIMANITE: Orthorhombic: Octahedral Al chains (6 -fold) are crosslinked by both Si and Al tetrahedra (4 -fold). • ANDALUSITE: Orthorhombic: 5 -coordinated Al; Same octahedral (6 fold) chains. • KYANITE: Triclinic: All the Al is octahedrally coordinated (6 - and 6 -fold). Andalusite Kyanite Sillimanite • Clearly, changes in structure are in response to changing P and T. Result is changes in Al coordination. • Phase transformations require rebonding of Al. Reconstructive polymorphism requires more energy than do displacive transformations. Metastability of these 3 are therefore important (Kinetic factors limit equilibrium attainment). • All 3 are VERY important metamorphic index minerals.

Aluminosilicate Minerals • 3 polymorphs of Al 2 Si. O 5 are important metamorphic minerals Andalusite Kyanite Sillimanite

Topaz • Aluminosilicate mineral as well, one oxygen substituted with OH, F • Al 2 Si. O 4(F, OH)2 • Where do you think Topaz forms? ?

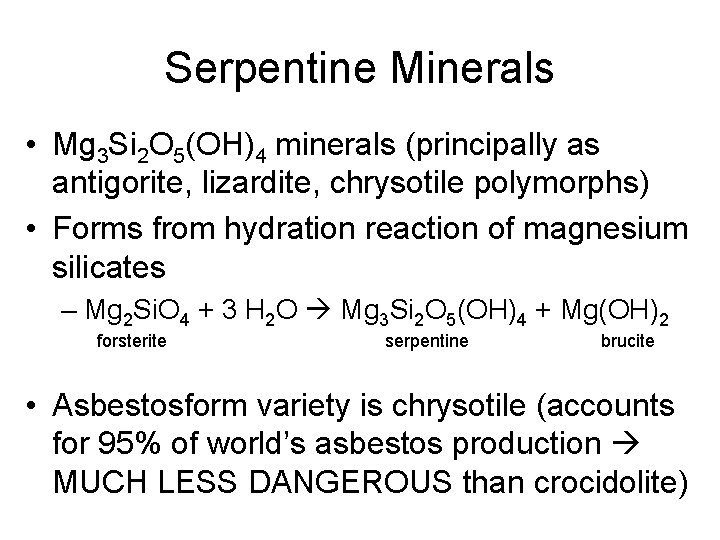
Serpentine Minerals • Mg 3 Si 2 O 5(OH)4 minerals (principally as antigorite, lizardite, chrysotile polymorphs) • Forms from hydration reaction of magnesium silicates – Mg 2 Si. O 4 + 3 H 2 O Mg 3 Si 2 O 5(OH)4 + Mg(OH)2 forsterite serpentine brucite • Asbestosform variety is chrysotile (accounts for 95% of world’s asbestos production MUCH LESS DANGEROUS than crocidolite)
![Phyllosilicates Yellow OH Serpentine Mg 3 Si 2 O 5 OH4 Tlayers and Phyllosilicates Yellow = (OH) Serpentine: Mg 3 [Si 2 O 5] (OH)4 T-layers and](https://slidetodoc.com/presentation_image_h2/8678cff53cc1f8f2d97ea108bc339e25/image-12.jpg)
Phyllosilicates Yellow = (OH) Serpentine: Mg 3 [Si 2 O 5] (OH)4 T-layers and triocathedral (Mg 2+) layers (OH) at center of T-rings and fill base of VI layer weak van der Waals bonds between T-O groups T O T O vdw

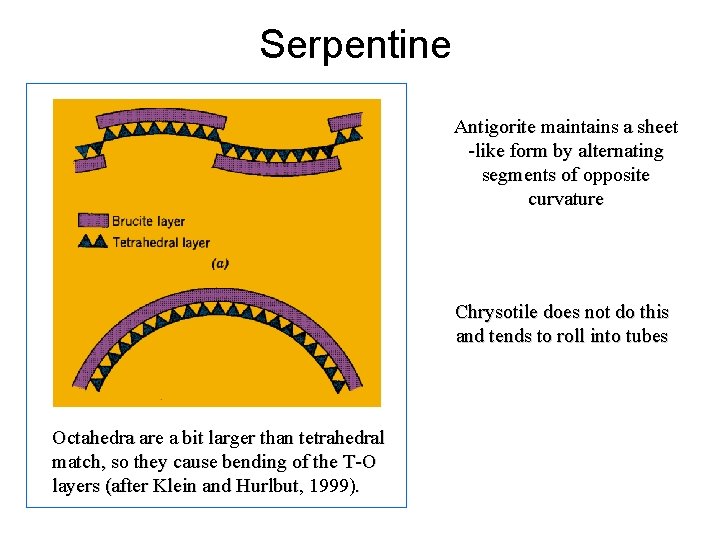
Serpentine Antigorite maintains a sheet -like form by alternating segments of opposite curvature Chrysotile does not do this and tends to roll into tubes Octahedra are a bit larger than tetrahedral match, so they cause bending of the T-O layers (after Klein and Hurlbut, 1999).

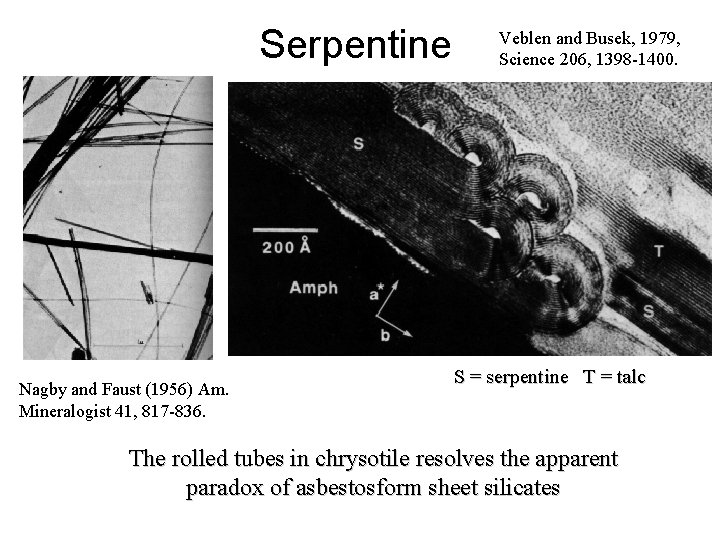
Serpentine Nagby and Faust (1956) Am. Mineralogist 41, 817 -836. Veblen and Busek, 1979, Science 206, 1398 -1400. S = serpentine T = talc The rolled tubes in chrysotile resolves the apparent paradox of asbestosform sheet silicates

Chlorite • Another phyllosilicate, a group of difficult to distinguish minerals • Typically green, and the dominant and characteristic mineral of greenschist facies rocks • Forms from the alteration of Mg-Fe silicates (pyroxenes, amphiboles, biotite, garnets) • Clinochlore, chamosite, pennantite, nimmite – end members • Chloritoid - Similar in appearance to chlorite, but different 2 V and relief

Prehnite-Pumpellyite • Low-grade metamorphic minerals • Minerals related to chlorite, form at slightly lower P-T conditions • Prehnite is also green, pumpellyite green too, varies based on Fe content • Prehnite + chlorite pumpellyite + quartz

Micas • Biotite and Muscovite are also important metamorphic minerals (muscovite often the principle component of schists) • Phlogopite – similar to biotite, but has little iron, forms from Mg-rich carbonate deposits and a common mineral in kimberlites (diamond-bearing material) • Sericite – white mica (similar to muscovite) – common product of plagioclase feldspar alteration at low grades

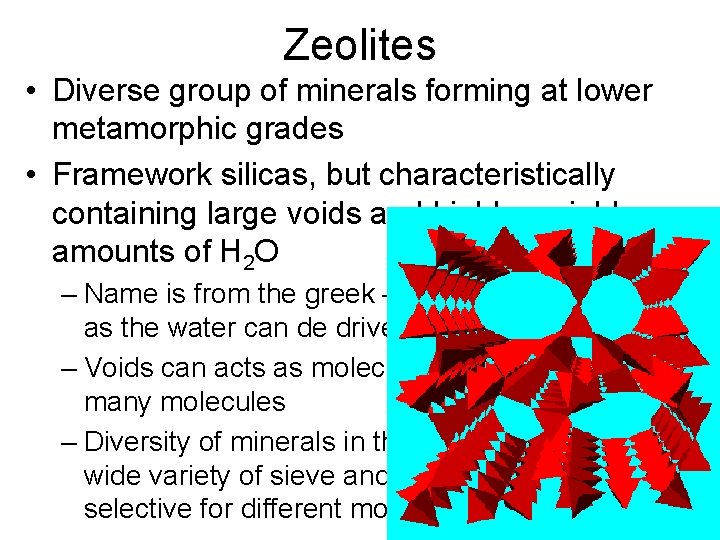
Zeolites • Diverse group of minerals forming at lower metamorphic grades • Framework silicas, but characteristically containing large voids and highly variable amounts of H 2 O – Name is from the greek – meaning to boil stone as the water can de driven off with heat – Voids can acts as molecular sieves and traps for many molecules – Diversity of minerals in this group makes a for a wide variety of sieve and trapping properties selective for different molecules

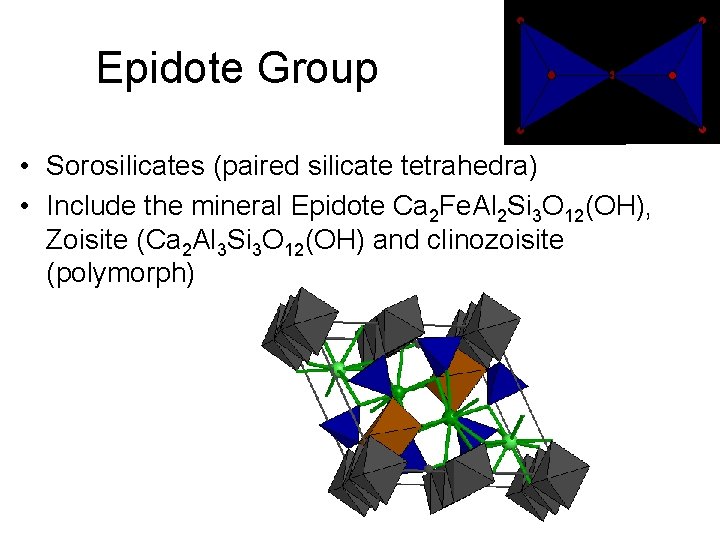
Epidote Group • Sorosilicates (paired silicate tetrahedra) • Include the mineral Epidote Ca 2 Fe. Al 2 Si 3 O 12(OH), Zoisite (Ca 2 Al 3 Si 3 O 12(OH) and clinozoisite (polymorph)
![Garnets Garnet A 23 B 32 Si O 43 Pyralspites B Al Garnets Garnet: A 2+3 B 3+2 [Si. O 4]3 “Pyralspites” - B = Al](https://slidetodoc.com/presentation_image_h2/8678cff53cc1f8f2d97ea108bc339e25/image-20.jpg)
Garnets Garnet: A 2+3 B 3+2 [Si. O 4]3 “Pyralspites” - B = Al Pyrope: Mg 3 Al 2 [Si. O 4]3 Almandine: Fe 3 Al 2 [Si. O 4]3 Spessartine: Mn 3 Al 2 [Si. O 4]3 “Ugrandites” - A = Ca Uvarovite: Ca 3 Cr 2 [Si. O 4]3 Grossularite: Ca 3 Al 2 [Si. O 4]3 Andradite: Ca 3 Fe 2 [Si. O 4]3 Occurrence: Mostly metamorphic Some high-Al igneous Also in some mantle peridotites Garnet (001) view blue = Si purple = A turquoise = B

Staurolite • Aluminosilicate - Fe 2 Al 9 Si 4 O 22(OH)2 • Similar structure to kyanite with tetrahedrally coordinated Fe 2+ easily replaced by Zn 2+ and Mg 2+ • Medium-grade metamorphic mineral, typically forms around 400 -500 C – chloritoid + quartz = staurolite + garnet – chloritoid + chlorite + muscovite = staurolite + biotite + quartz + water • Degrades to almandine (garnet at higher T) – staurolite + muscovite + quartz = almandine + aluminosilicate + biotite + water

Metamorphic chain silicates • Actinolite and tremolite are chain silicates derived from dolomite and quartz and common in low-mid grade metamorphic rocks • Riebeckite and Glaucophane are also chain silicates – higher grade minerals, often a blue color • These minerals usually lower P, higher T conditions

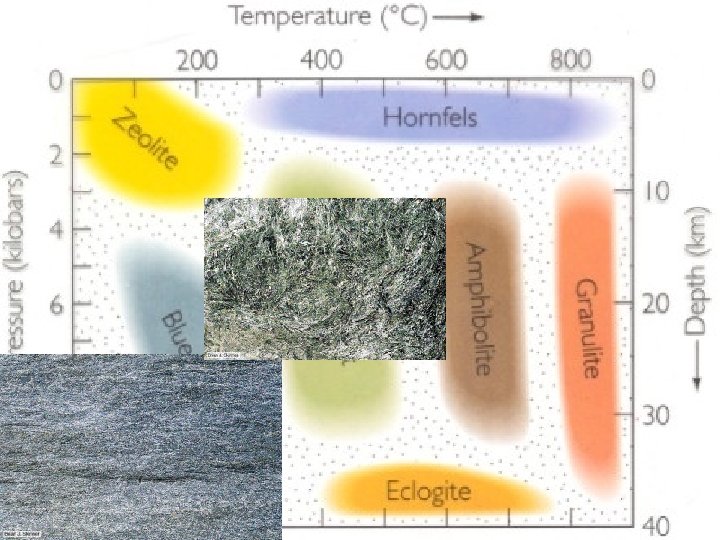
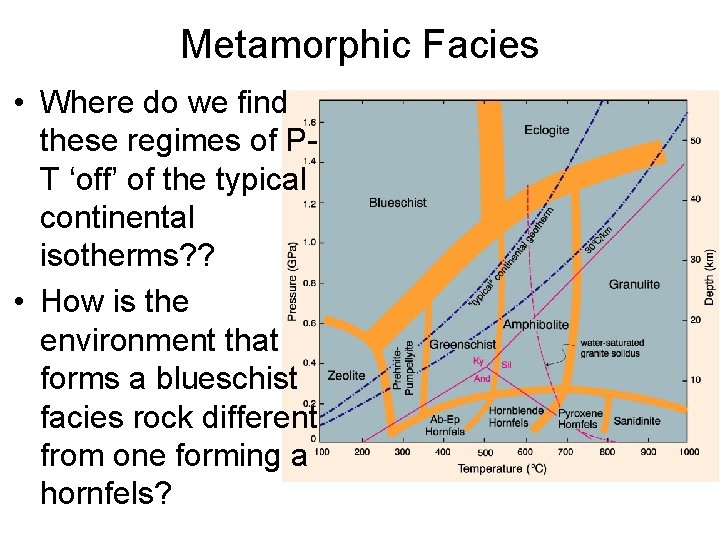
Metamorphic Facies • Where do we find these regimes of PT ‘off’ of the typical continental isotherms? ? • How is the environment that forms a blueschist facies rock different from one forming a hornfels?

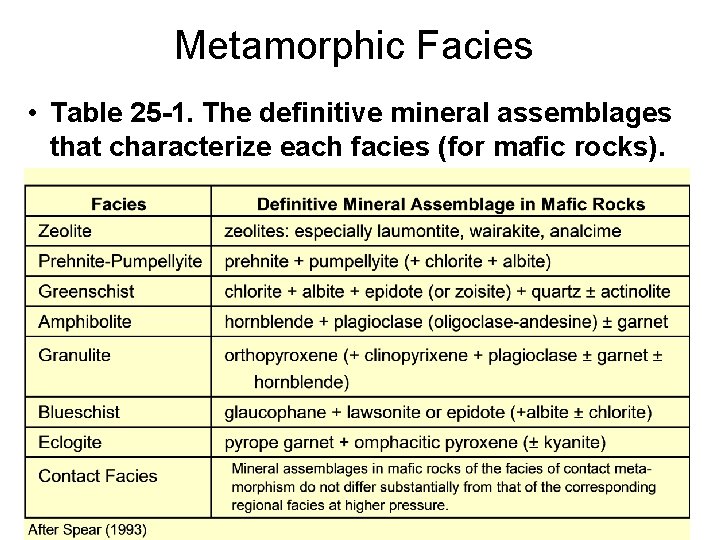
Metamorphic Facies • Table 25 -1. The definitive mineral assemblages that characterize each facies (for mafic rocks).

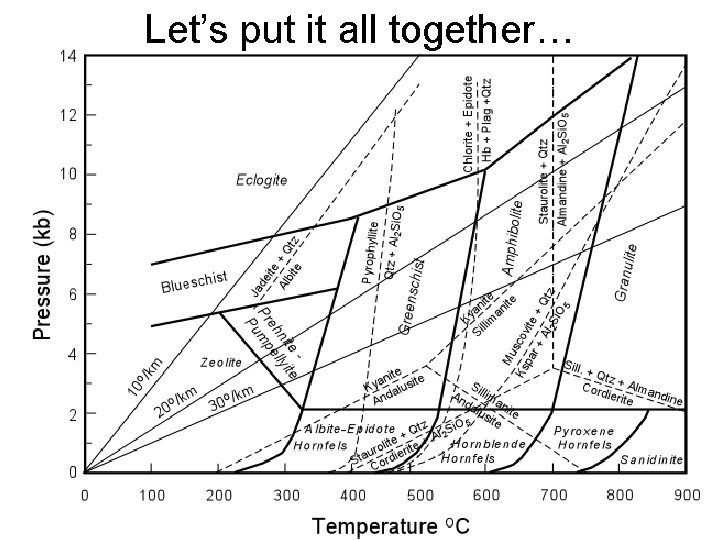
Let’s put it all together…

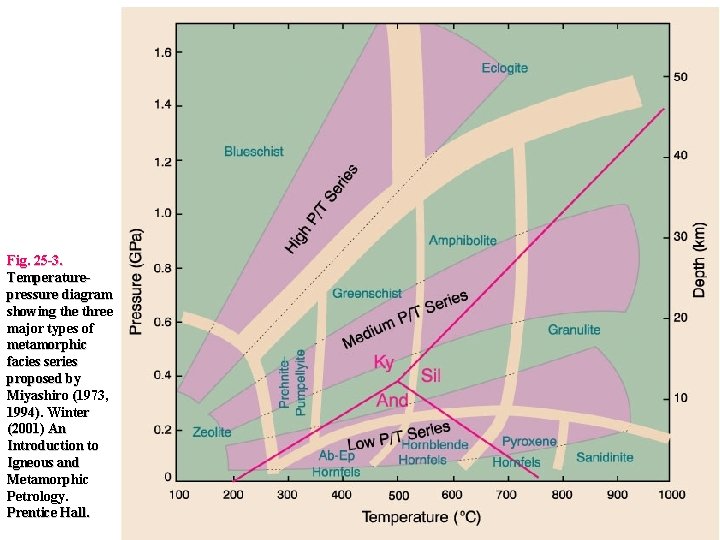
Facies Series • Miyashiro (1961) initially proposed five facies series, most of them named for a specific representative “type locality” The series were: 1. Contact Facies Series (very low-P) 2. Buchan or Abukuma Facies Series (low-P regional) 3. Barrovian Facies Series (medium-P regional) 4. Sanbagawa Facies Series (high-P, moderate-T) 5. Franciscan Facies Series (high-P, low T)

Fig. 25 -3. Temperaturepressure diagram showing the three major types of metamorphic facies series proposed by Miyashiro (1973, 1994). Winter (2001) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall.

Isograds • Lines (on a map) or Surfaces (in the 3 D world) marking the appearance or disappearance of the Index minerals in rocks of appropriate composition e. g. the ‘garnet-in isograd’; the ‘stauroliteout isograd’ Complicated by the fact that most of these minerals are solid solutions

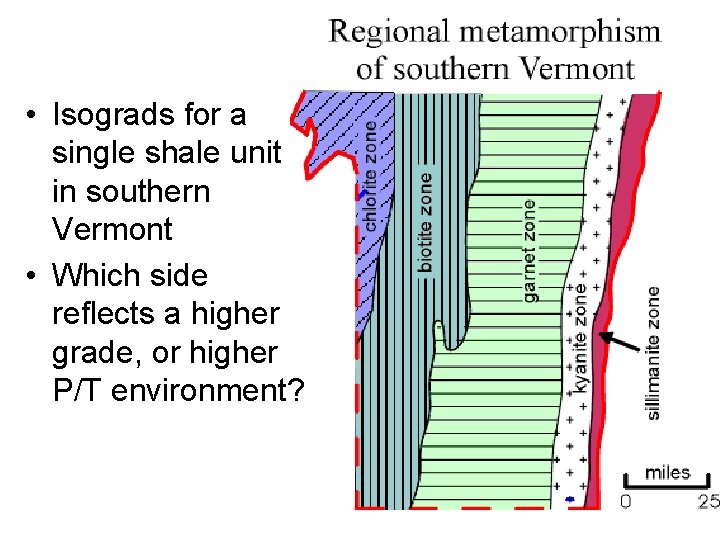
• Isograds for a single shale unit in southern Vermont • Which side reflects a higher grade, or higher P/T environment?