The Periodic Table What Does Periodic Mean l
The Periodic Table

What Does Periodic Mean? l Periodic means repeated in a pattern l Think about a calendar: l Weeks are periodic – they repeat every 7 days

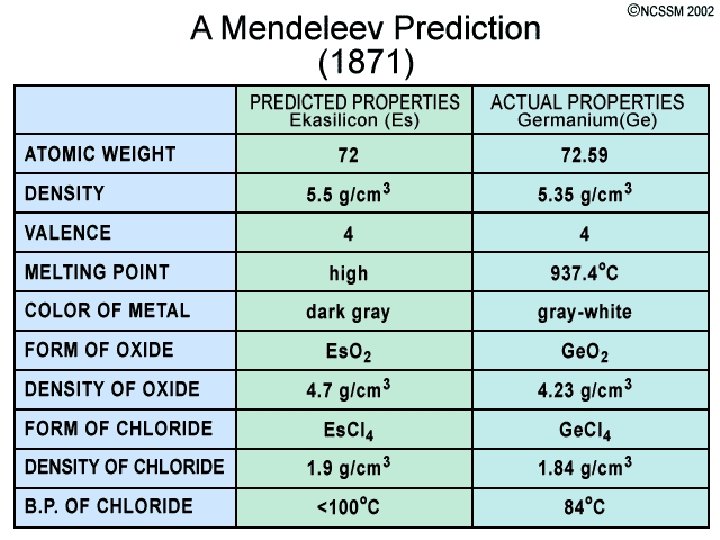
Dimitri Mendeleev (1834 - 1907) l Created the first way to organize all known elements l Based his table on the pattern of chemical properties
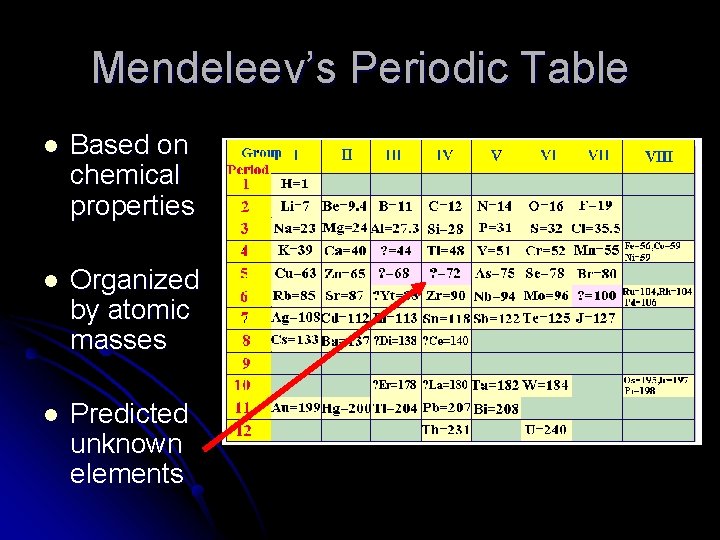
Mendeleev’s Periodic Table l Based on chemical properties l Organized by atomic masses l Predicted unknown elements

Henry Moseley (1887 – 1915) l His work led to a reorganization of Mendeleev’s table by atomic number l Killed by a sniper in World War One at age 27
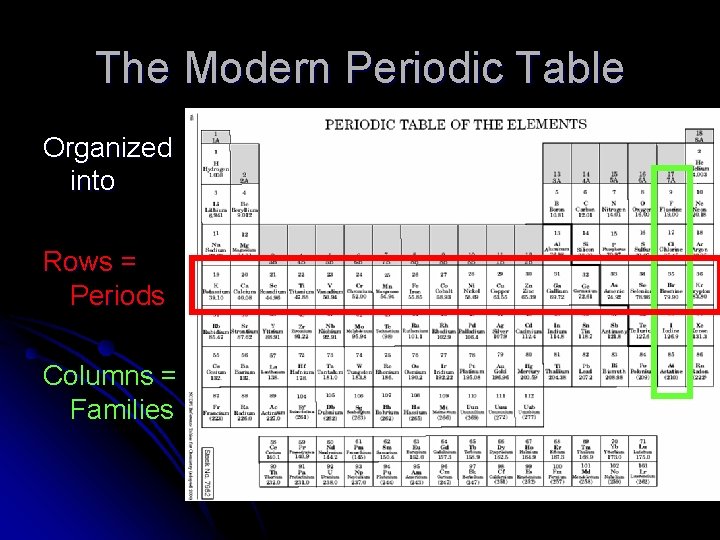
The Modern Periodic Table Organized into Rows = Periods Columns = Families

Periods and Families l The families are numbered at the top of your periodic table in your reference table l Take a pen and number the periods l Number one is the row with Hydrogen l Number seven is the row with Francium
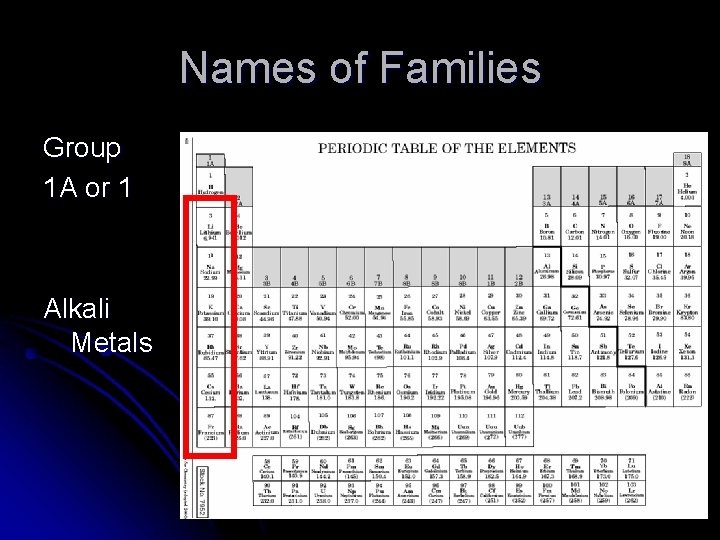
Names of Families Group 1 A or 1 Alkali Metals
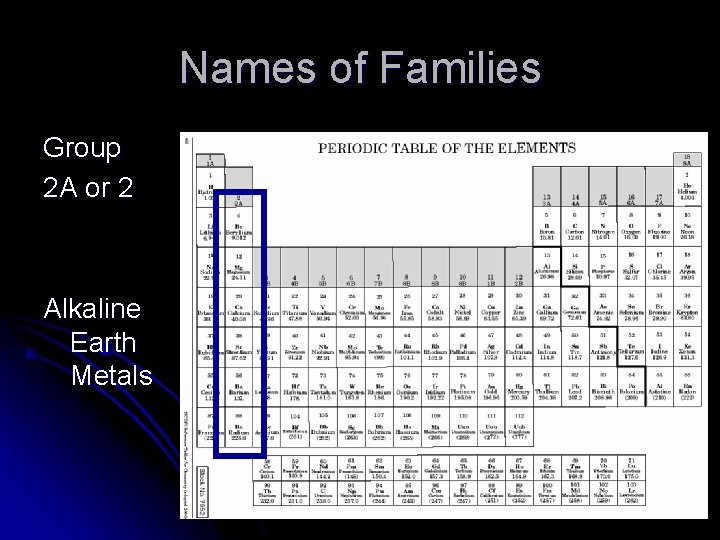
Names of Families Group 2 A or 2 Alkaline Earth Metals
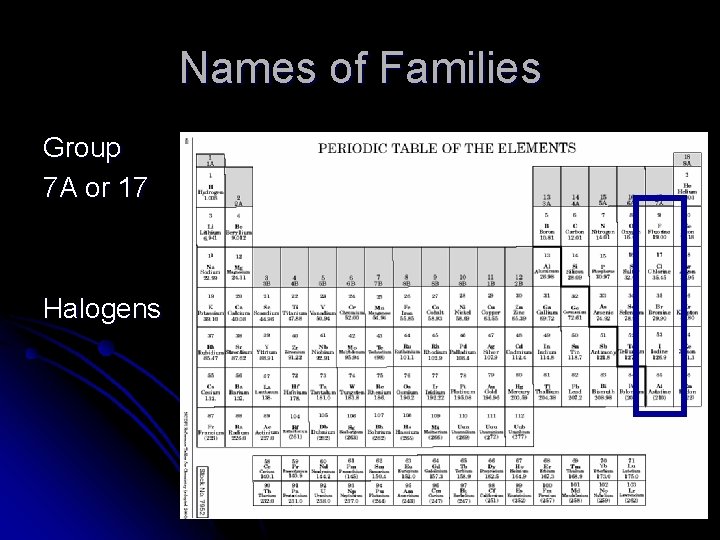
Names of Families Group 7 A or 17 Halogens
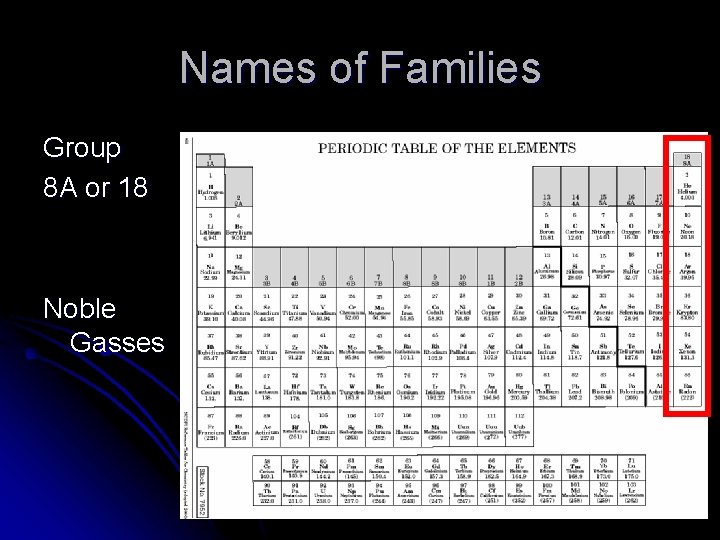
Names of Families Group 8 A or 18 Noble Gasses
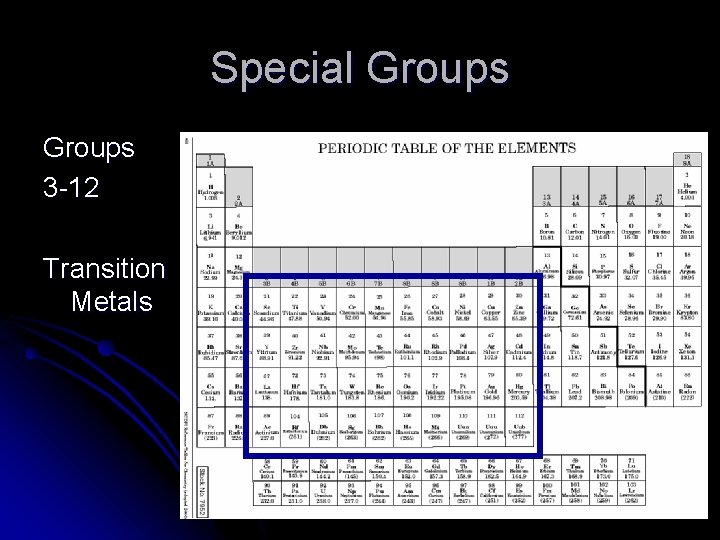
Special Groups 3 -12 Transition Metals
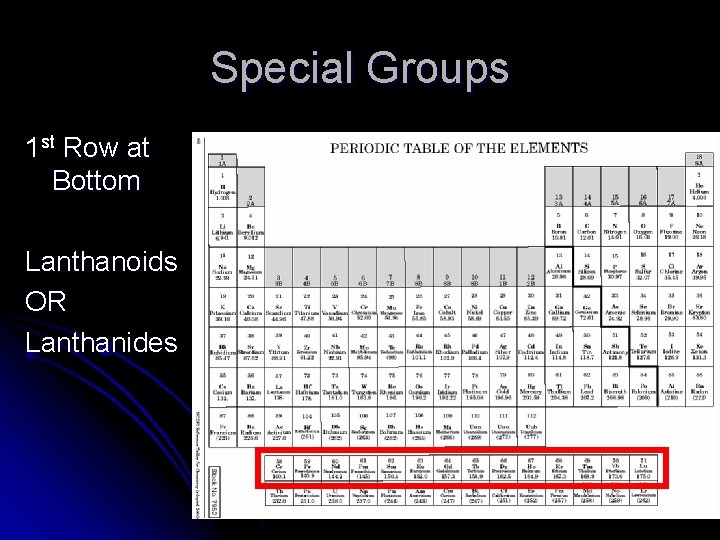
Special Groups 1 st Row at Bottom Lanthanoids OR Lanthanides
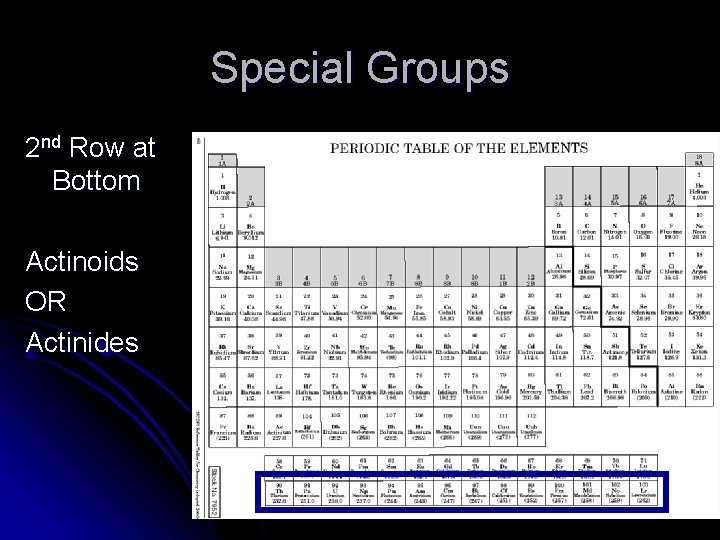
Special Groups 2 nd Row at Bottom Actinoids OR Actinides
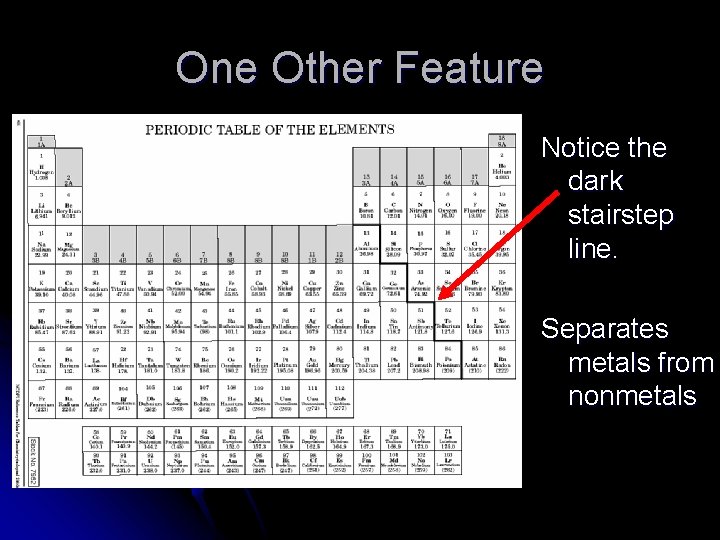
One Other Feature Notice the dark stairstep line. Separates metals from nonmetals
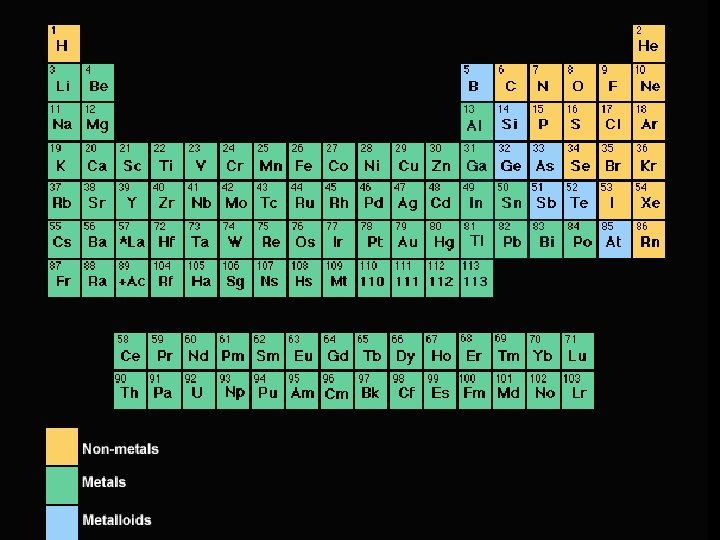

Metals l To the left of the stair-step line l Typically solids with high melting points l Good conductors of heat and electricity l Malleable – can be formed into a different shape l Ductile – can be drawn into wires

Metals l As you move left across the periodic table, metals become more reactive l As you move down a group (family), metals become more reactive l Remember the video about cesium? l Where are the most reactive elements on the periodic table?

Metalloids l Touching the stair-step line l Have properties in between metals and nonmetals l Semiconductors – Think silicon in computer chips

Nonmetals l To the right of the stair-step line l Typically gasses with low melting points l Very poor conductors of heat and electricity l Brittle – break rather than change shape

Nonmetals l As you move right across the periodic table, nonmetals become more reactive l As you move down a group (family), nonmetals become less reactive l Where are the most reactive nonmetals?

Exit Ticket l 1. 2. 3. 4. On the index card, please answer the following questions: Which group is the halogens? What is the most reactive nonmetal? What is the most reactive metal? List two metalloids.
- Slides: 23