The Periodic Table Topic 5 Click for song
The Periodic Table- Topic 5 Click for song SMB, Periodic Table Notes 2011 I II III

Click on pix for history SMB, Periodic Table Notes 2011 2

I. HISTORY A. Dmitri Mendeleev (1869, Russian) z Organized elements by increasing ATOMIC MASS. q. Elements with similar chemical properties were grouped together. q. There were some discrepancies. SMB, Periodic Table Notes 2011 3

B. Henry Moseley q ORGANIZED ELEMENTS BY INCREASING ATOMIC NUMBER. q Resolved discrepancies in Mendeleev’s arrangement. SMB, Periodic Table Notes 2011 4
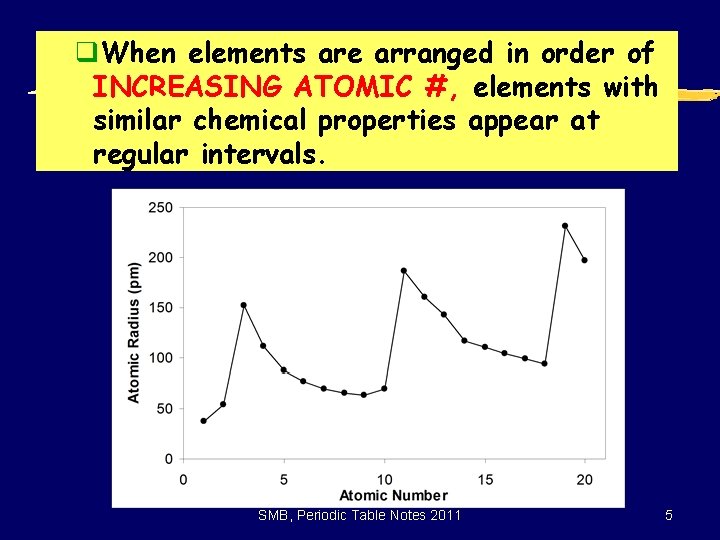
q. When elements are arranged in order of INCREASING ATOMIC #, elements with similar chemical properties appear at regular intervals. SMB, Periodic Table Notes 2011 5
II. ORGANIZATION OF THE ELEMENTS A. Arrangement of Table 1. Horizontal rows q. Called PERIODS q. All elements in the same period have the same number of ENERGY LEVELS in their atomic structure SMB, Periodic Table Notes 2011 6
2. Vertical Columns a) Called GROUPS OR FAMILIES b) All elements in the same group have the same number of VALENCE ELECTRONS, therefore lose or gain the SAME number of electrons, form similar CHEMICAL FORMULAS and have similar CHEMICAL PROPERTIES ex. XCl 2 Group 2: y. Remember: When +2 Cl -1 = Be. Cl Be writing formulas, use 2 the criss-cross rule Mg +2 Cl -1 = Mg. Cl 2 to cancel out oxidation states SMB, Periodic Table Notes 2011 7
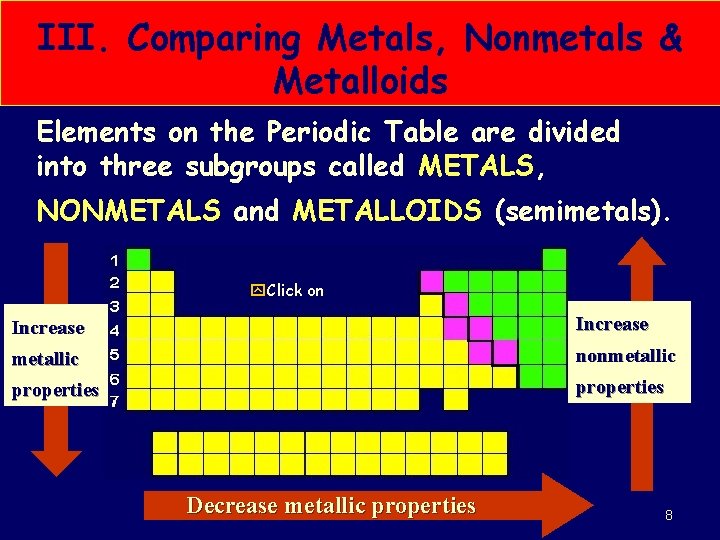
III. Comparing Metals, Nonmetals & Metalloids Elements on the Periodic Table are divided into three subgroups called METALS, NONMETALS and METALLOIDS (semimetals). y. Click on Increase metallic nonmetallic properties Decrease metallic properties SMB, Periodic Table Notes 2011 8

METALS: located on the LEFT SIDE of the periodic table (except H); MORE THAN 2/3 of all elements 1. Chemical properties q tend to LOSE ELECTRONS EASILY q have LOW IONIZATION ENERGY (energy needed to remove electrons) q Metallic character INCREASES as ionization energy decreases. q have LOW ELECTRON AFFINITY (attraction for electrons) q form POSITIVE IONS when combining with other atoms q FRANCIUM most reactive metal: See Table J http: //castlelearning. com/review/reference/chem%20 table%20 j. htm SMB, Periodic Table Notes 2011 9
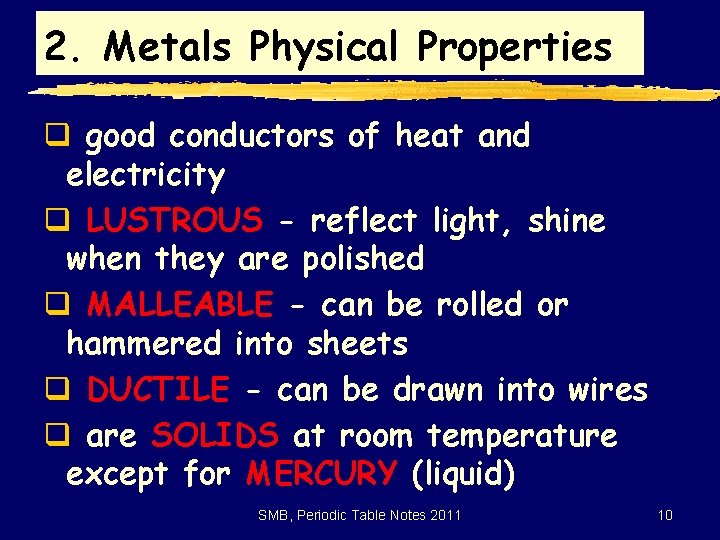
2. Metals Physical Properties q good conductors of heat and electricity q LUSTROUS - reflect light, shine when they are polished q MALLEABLE - can be rolled or hammered into sheets q DUCTILE - can be drawn into wires q are SOLIDS at room temperature except for MERCURY (liquid) SMB, Periodic Table Notes 2011 10

located on the right side of the B. NONMETALS periodic table (except for Noble gases) 1. Chemical properties q tend to GAIN electrons to form NEGATIVE IONS q have high electron affinities (electronegativity) q produce COVALENT bonds by SHARING electrons with other nonmetals q FLUORINE most reactive nonmetal: see Table JSMB, Periodic Table Notes 2011 11
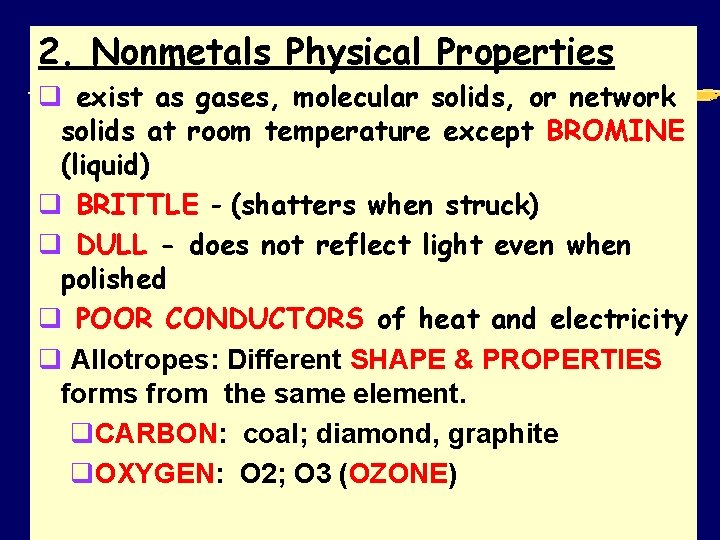
2. Nonmetals Physical Properties q exist as gases, molecular solids, or network solids at room temperature except BROMINE (liquid) q BRITTLE - (shatters when struck) q DULL - does not reflect light even when polished q POOR CONDUCTORS of heat and electricity q Allotropes: Different SHAPE & PROPERTIES forms from the same element. q. CARBON: coal; diamond, graphite q. OXYGEN: O 2; O 3 (OZONE) SMB, Periodic Table Notes 2011 12
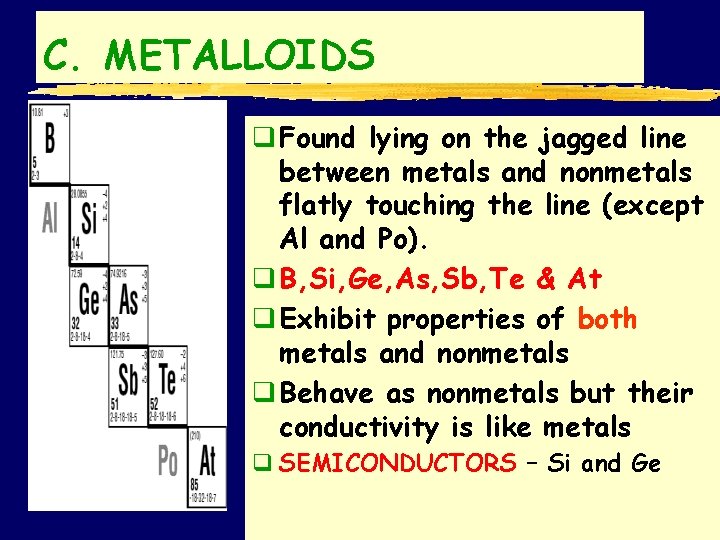
C. METALLOIDS q Found lying on the jagged line between metals and nonmetals flatly touching the line (except Al and Po). q B, Si, Ge, As, Sb, Te & At q Exhibit properties of both metals and nonmetals q Behave as nonmetals but their conductivity is like metals q SEMICONDUCTORS – Si and Ge SMB, Periodic Table Notes 2011 13
IV. Periodic Trends – use Table S A. Periodic Law q When elements are arranged in order of increasing atomic #, elements with similar properties appear at regular intervals. q http: //castlelearning. com/review/reference/chem%20 table%20 s. htm SMB, Periodic Table Notes 2011 14

1) Ionization Energy z. Energy needed to remove the most loosely bound electron from a neutral gaseous atom z X + energy X+ + e-
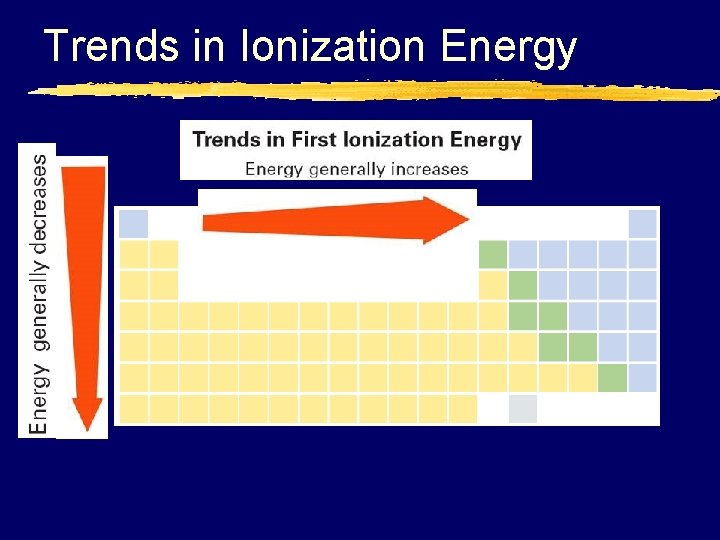
6. 3 Trends in Ionization Energy

Trends in Ionization Energy z. IE increases as you move across a period y. Why? x. The nuclear charge (atomic #) is increasing therefore greater attraction of the nucleus for electrons hence harder to remove an electron

Trends in Ionization Energy z. IE decreases as you move down a group y. Why? x. Atom size increases making the outermost electron farther away from the nucleus therefore making it easier to remove x. Shielding increases
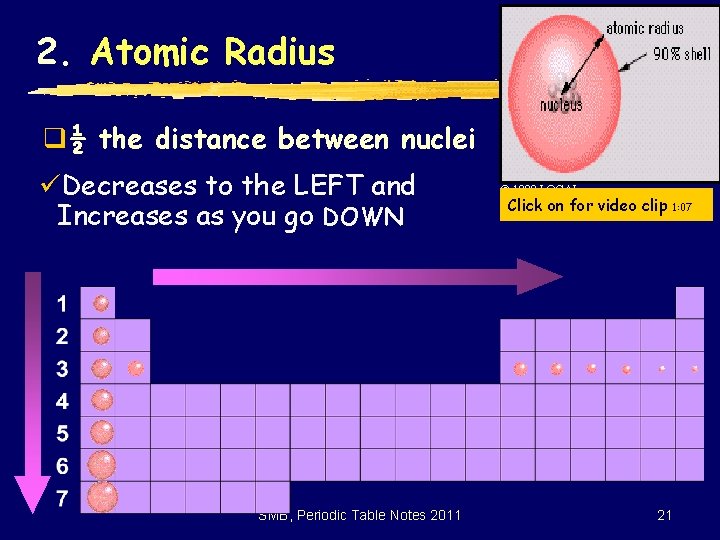
2. Atomic Radius q½ the distance between nuclei üDecreases to the LEFT and Increases as you go DOWN SMB, Periodic Table Notes 2011 © 1998 LOGAL Click on for video clip 1: 07 21

Atomic Radius cont. q. Why is it larger going down? üHigher energy levels have larger orbitals üShielding - core e- block the attraction between the nucleus and the valence eq. Why is it smaller to the right? üIncreased nuclear charge without additional shielding pulls e- in tighter SMB, Periodic Table Notes 2011 22

Comparison? ? ? q. Why is the ionization energy opposite that of atomic radius? üIn small atoms, e- are close to the nucleus where the attraction is stronger q. Why small jumps within each group? üStable e- configurations don’t want to lose e. SMB, Periodic Table Notes 2011 23
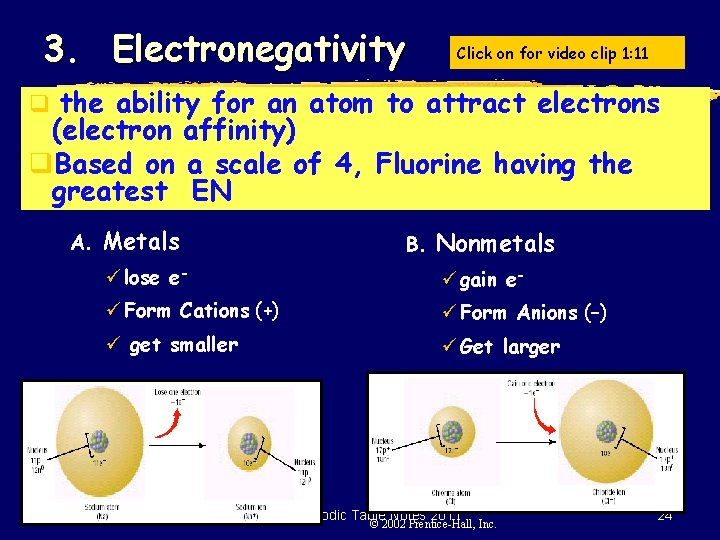
3. Electronegativity Click on for video clip 1: 11 q the ability for an atom to attract electrons (electron affinity) q. Based on a scale of 4, Fluorine having the greatest EN A. Metals B. Nonmetals ü lose e- ü gain e- ü Form Cations (+) ü Form Anions (–) ü get smaller ü Get larger SMB, Periodic Table Notes 2011 © 2002 Prentice-Hall, Inc. 24
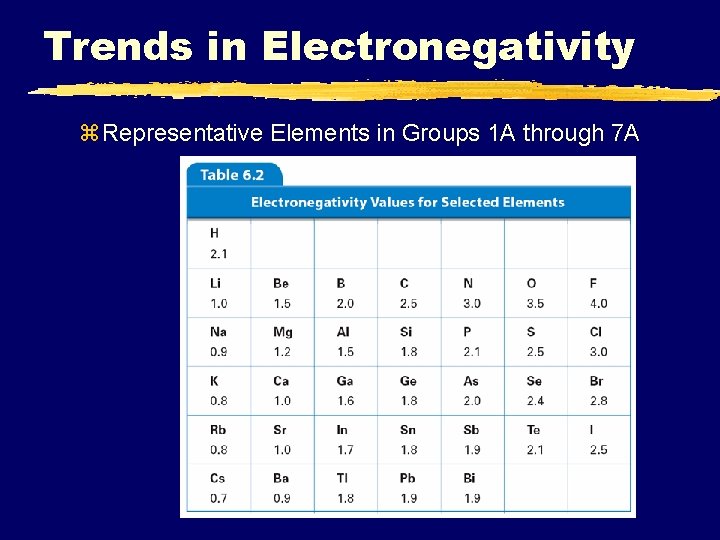
6. 3 Trends in Electronegativity z Representative Elements in Groups 1 A through 7 A
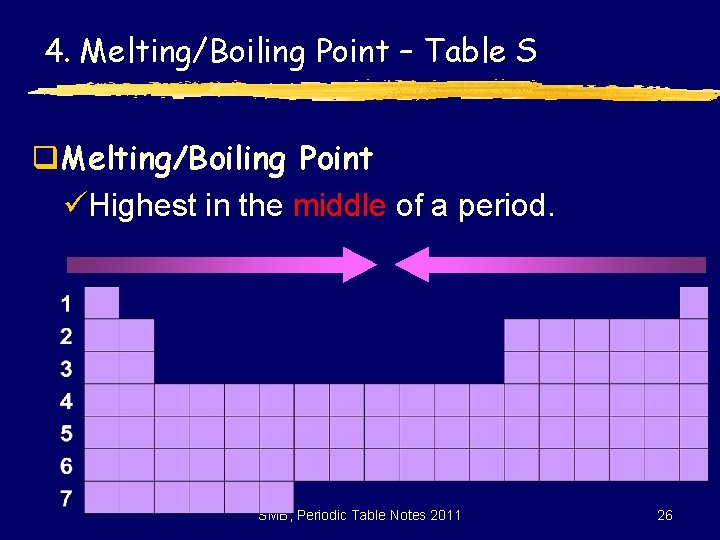
4. Melting/Boiling Point – Table S q. Melting/Boiling Point üHighest in the middle of a period. SMB, Periodic Table Notes 2011 26

Periodic Trends Summary (use reference Table S for data comparison) Trend Across a period Down a group (L to R) Ionization energy increases decreases Electronegativity increases decreases Atomic radii decreases increases Metallic properties decreases increases y Click on for video clip 4: 41 SMB, Periodic Table Notes 2011 27
IV. Classification q. Alkali Metals q. Alkaline Earth Metals q. Transition Metals q. Halogens q. Noble Gases SMB, Periodic Table Notes 2011 Click for song 28

Group 1: Alkali Metals qextremely reactive (not found free in nature) q form stable ionic compounds qreact with water to form a base qreact with air to form oxides qreact with acids to form salts Click on for video clip SMB, Periodic Table Notes 2011 29
Group 2: Alkaline Earth Metals qreactive (not found free in nature) form stable ionic compounds qreact with water to form a base qreact with air to form oxides q react with acids to form salts Click on for video clip SMB, Periodic Table Notes 2011 30
Groups 3 -11: Transition Metals qmultiple positive oxidation states q. Lose electrons from two outermost energy levels q. Ions form colored solutions SMB, Periodic Table Notes 2011 31
Group 15 – unique features q. Members range from typical nonmetals (nitrogen and phosphorus) through metalloids (arsenic and antimony) to metals (bismuth) q Nitrogen q. Forms stable diatomic molecules with a triple bond q. Component of protein q. Forms some unstable compounds that are used as explosives q Phosphorus q. Component of nucleic acids (DNA, RNA) q. More reactive than nitrogen at room temperature SMB, Periodic Table Notes 2011 32

Group 16 – unique Features q. Members range from typical nonmetals (oxygen and sulfur) through metalloids (selenium and tellurium) to metals (polonium) q. Solids except oxygen q Oxygen can exist as O 2 and O 3 (it is an allotrope) q. Polonium is radioactive SMB, Periodic Table Notes 2011 33
Group 17: Halogens q very reactive nonmetals - high electronegativity q not found free in nature q form diatomic molecules when free q react with metals to form salts (halides) q Found in all three phases (s, l, g) due to differences in Van der Waals forces (these are weak) SMB, Periodic Table Notes 2011 34

Group 18: Noble Gases q. Have complete outer shells q. Almost inert (not reactive); stable q. Krypton, xenon, and radon form compounds with oxygen and fluorine q. Referred to as monatomic gases SMB, Periodic Table Notes 2011 35
- Slides: 33