The Periodic Table Topic 1 5 Color this

+ The Periodic Table Topic 1. 5
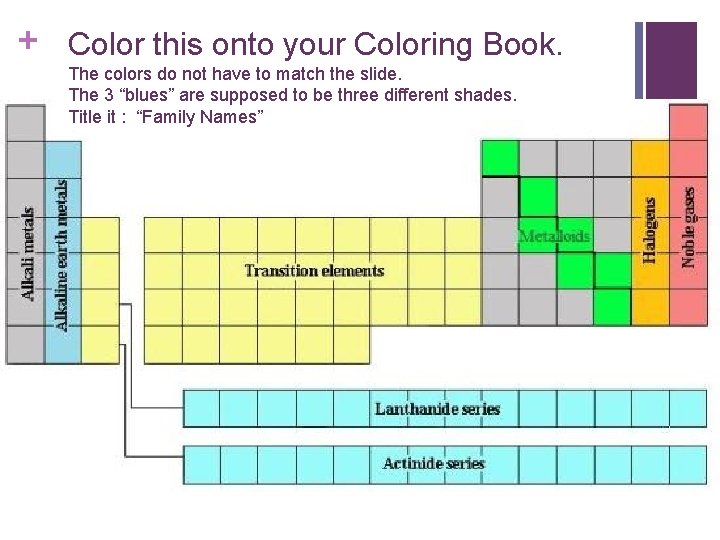
+ Color this onto your Coloring Book. The colors do not have to match the slide. The 3 “blues” are supposed to be three different shades. Title it : “Family Names”
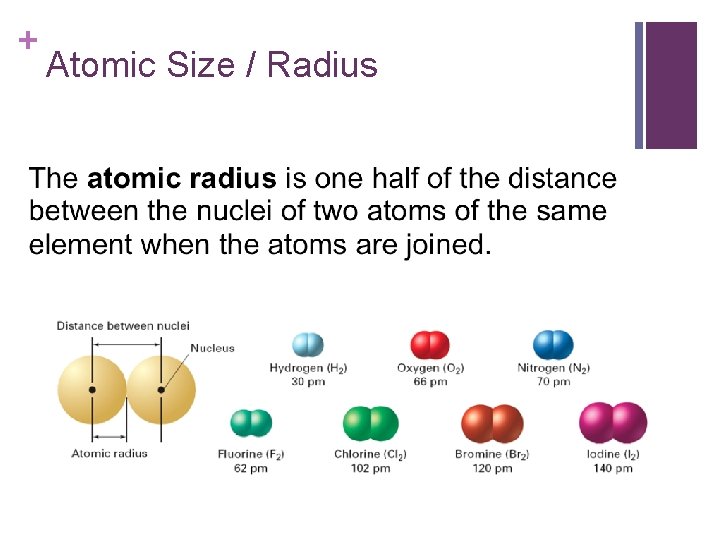
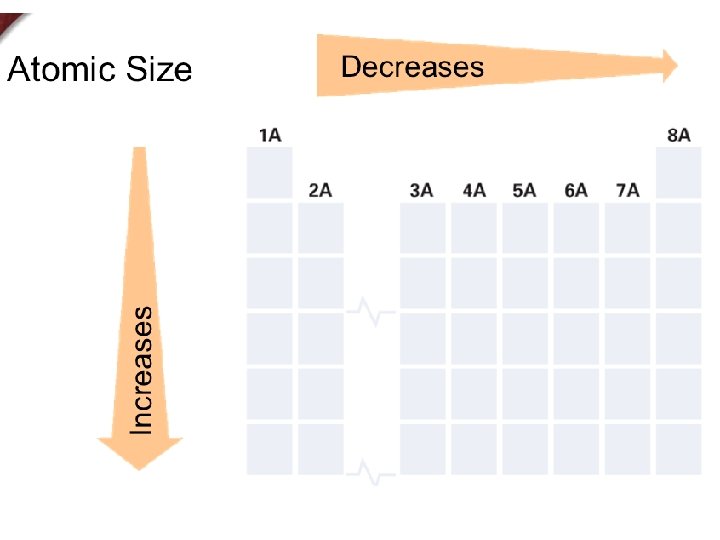
+ Atomic Size / Radius
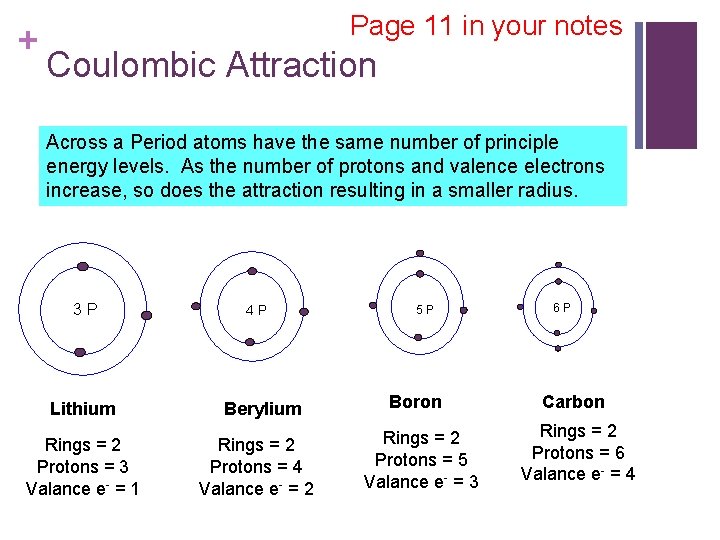
+ Page 11 in your notes Coulombic Attraction Across a Period atoms have the same number of principle energy levels. As the number of protons and valence electrons increase, so does the attraction resulting in a smaller radius. 3 P Lithium Rings = 2 Protons = 3 Valance e- = 1 4 P Berylium Rings = 2 Protons = 4 Valance e- = 2 5 P Boron Rings = 2 Protons = 5 Valance e- = 3 6 P Carbon Rings = 2 Protons = 6 Valance e- = 4
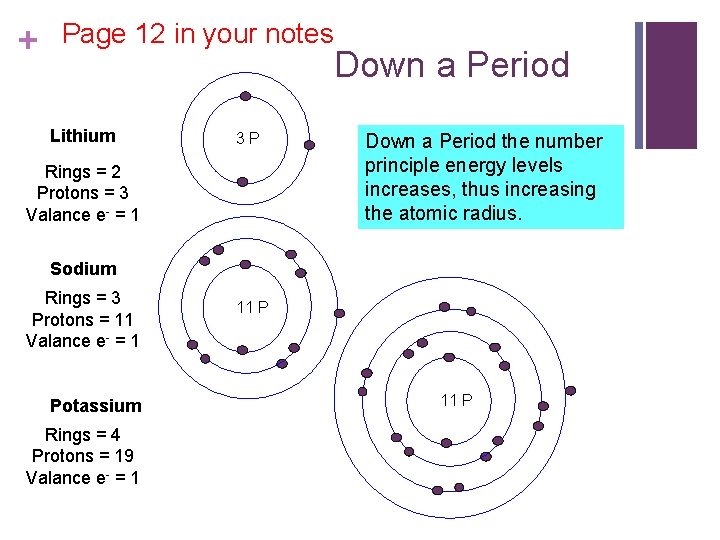
+ Page 12 in your notes Lithium 3 P Rings = 2 Protons = 3 Valance e- = 1 Down a Period the number principle energy levels increases, thus increasing the atomic radius. Sodium Rings = 3 Protons = 11 Valance e- = 1 Potassium Rings = 4 Protons = 19 Valance e- = 1 11 P
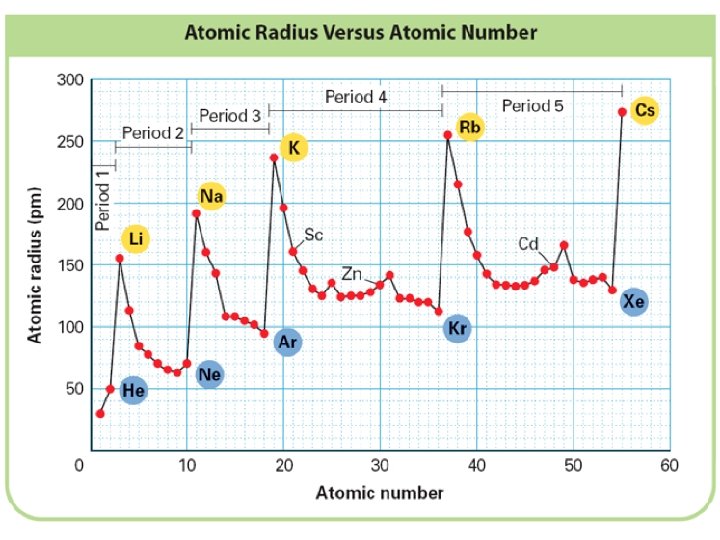
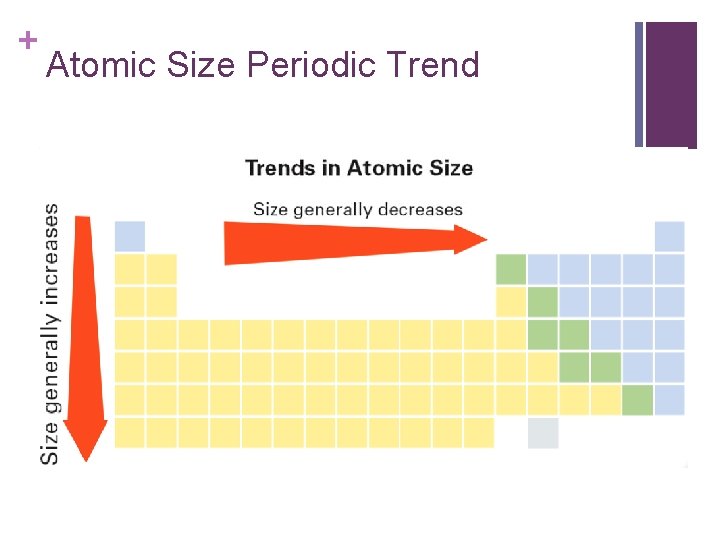
+ Atomic Size Periodic Trend
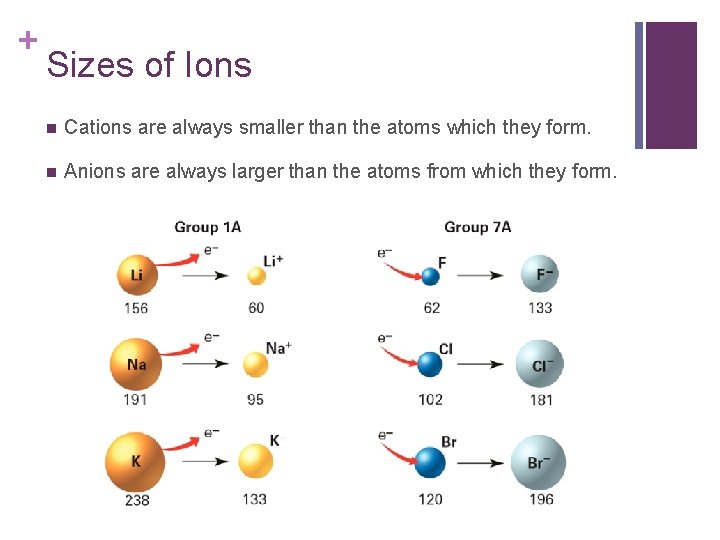
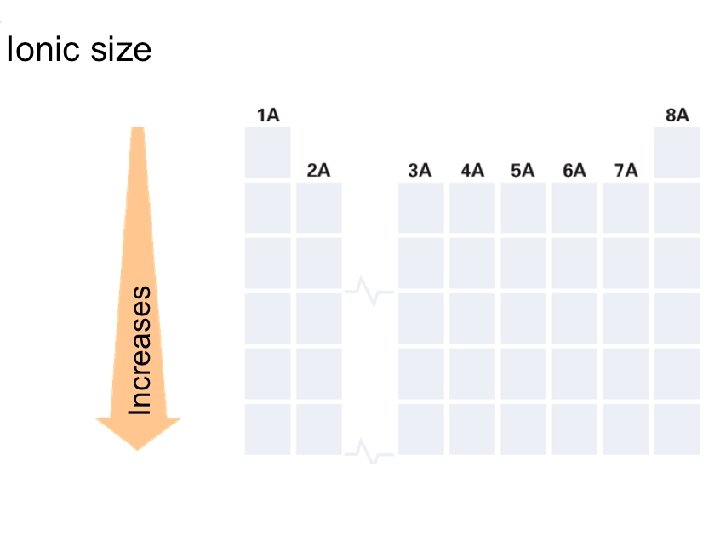
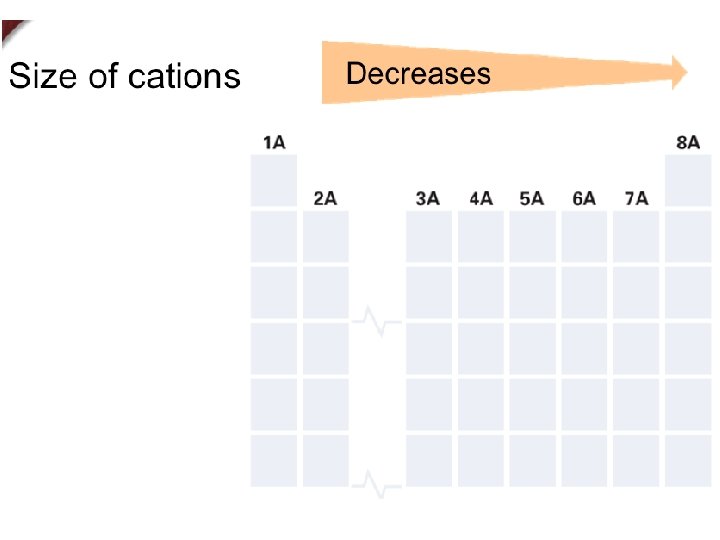
+ Sizes of Ions n Cations are always smaller than the atoms which they form. n Anions are always larger than the atoms from which they form.
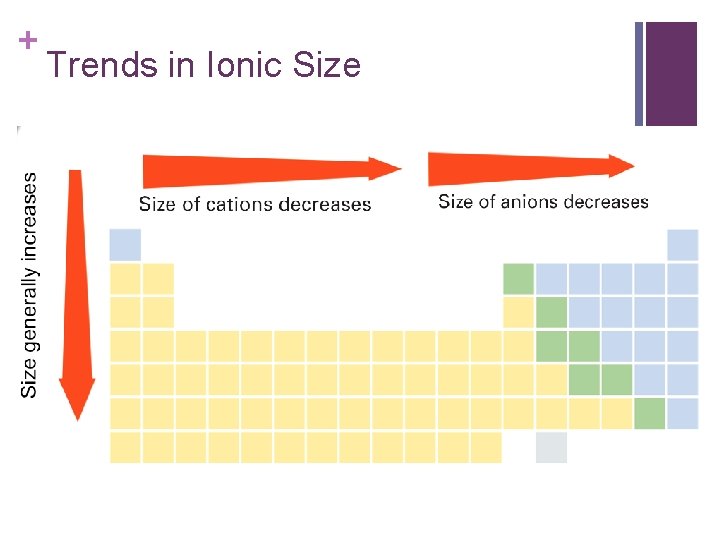
+ Trends in Ionic Size
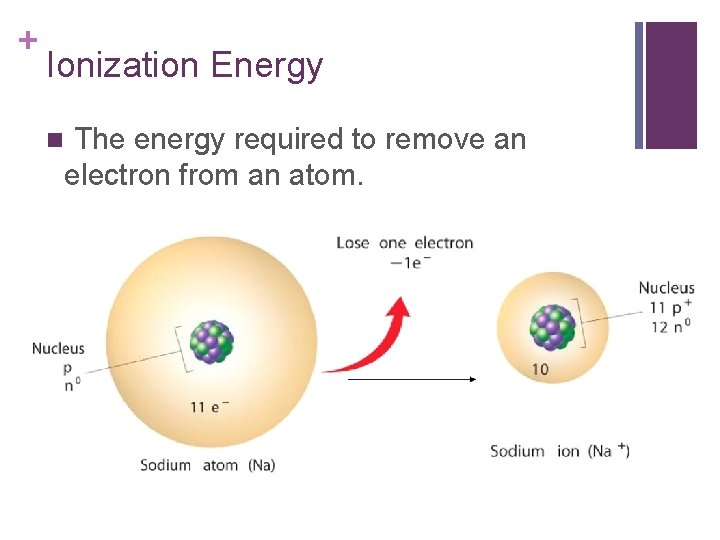
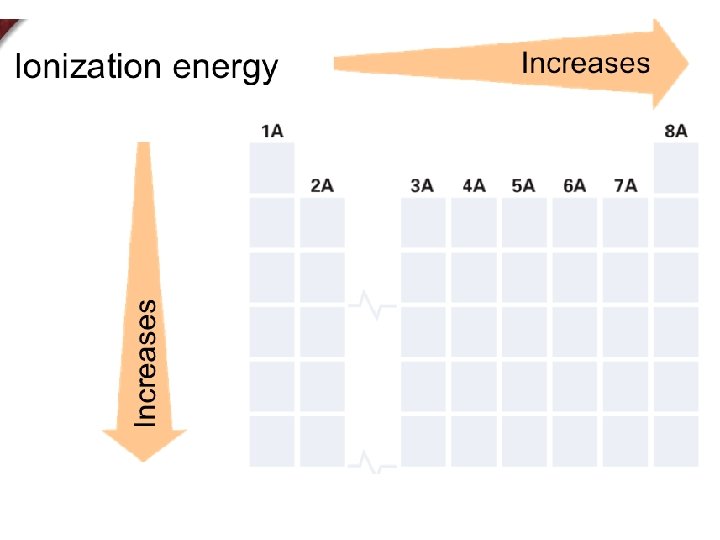
+ Ionization Energy The energy required to remove an electron from an atom. n
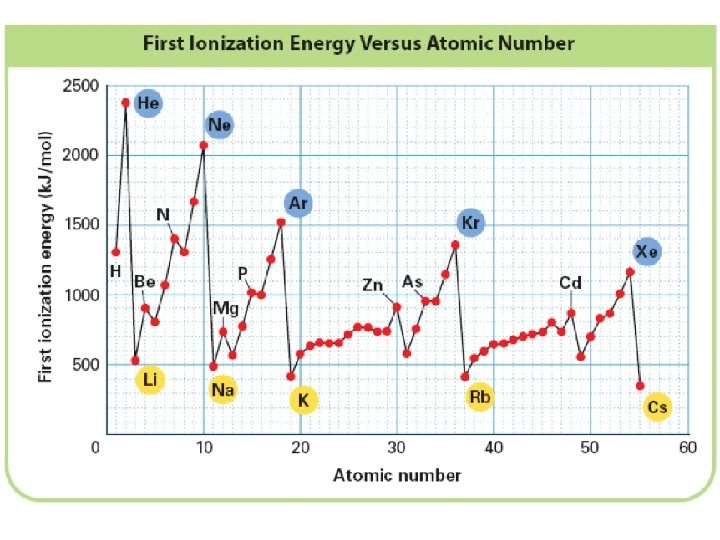
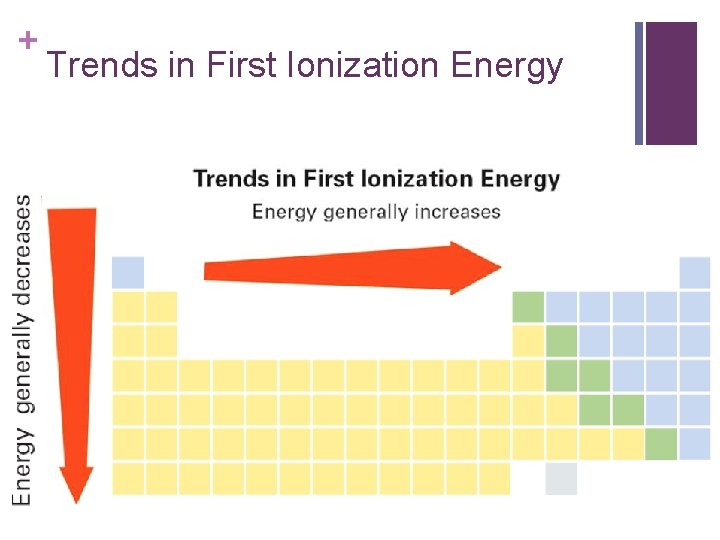
+ Trends in First Ionization Energy

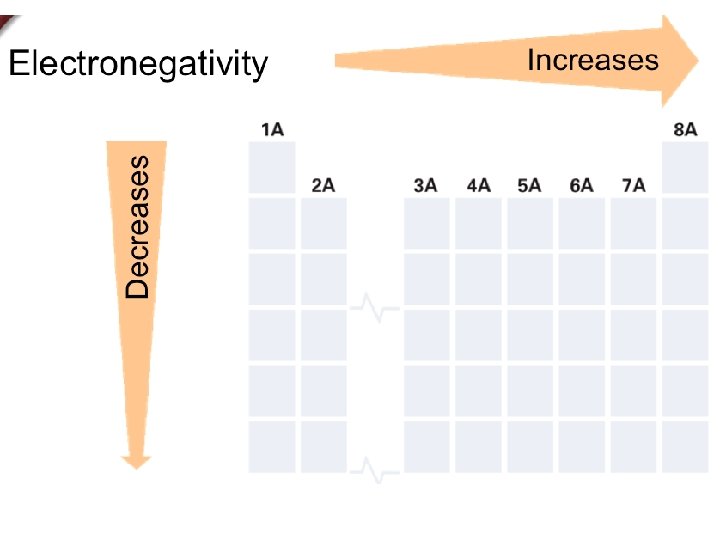
+ Electronegativity n The ability of an atom to attract electrons. n These values are derived from ionization energy and follow the same trend.
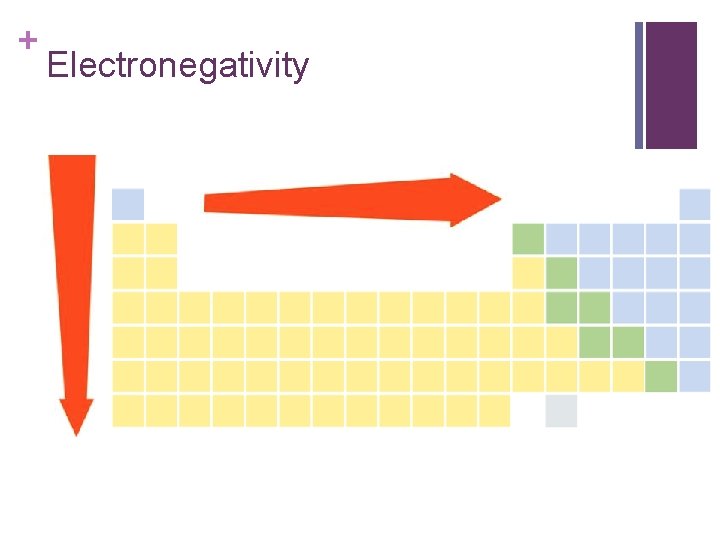
+ Electronegativity
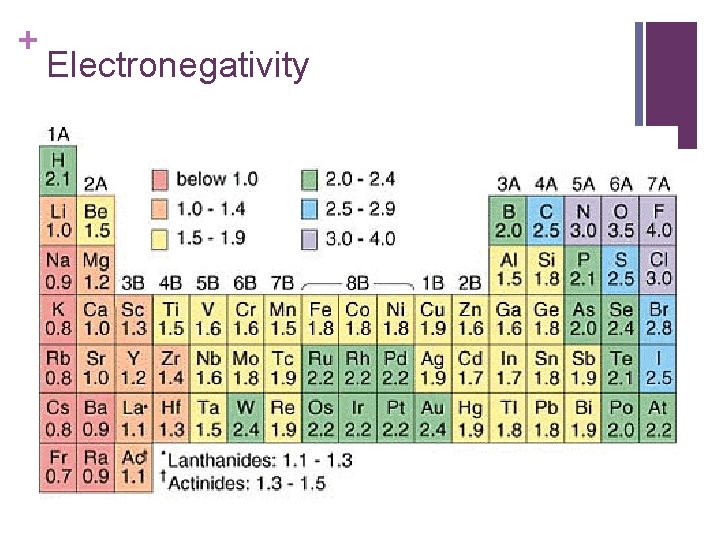
+ Electronegativity
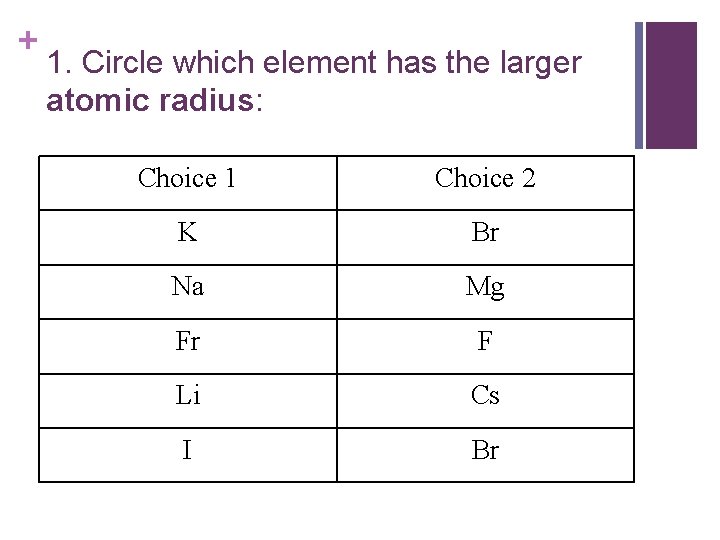
+ 1. Circle which element has the larger atomic radius: Choice 1 Choice 2 K Br Na Mg Fr F Li Cs I Br
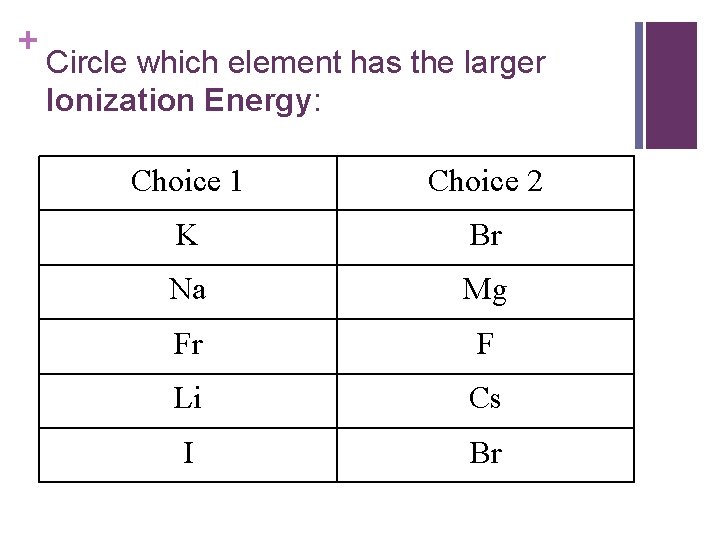
+ Circle which element has the larger Ionization Energy: Choice 1 Choice 2 K Br Na Mg Fr F Li Cs I Br
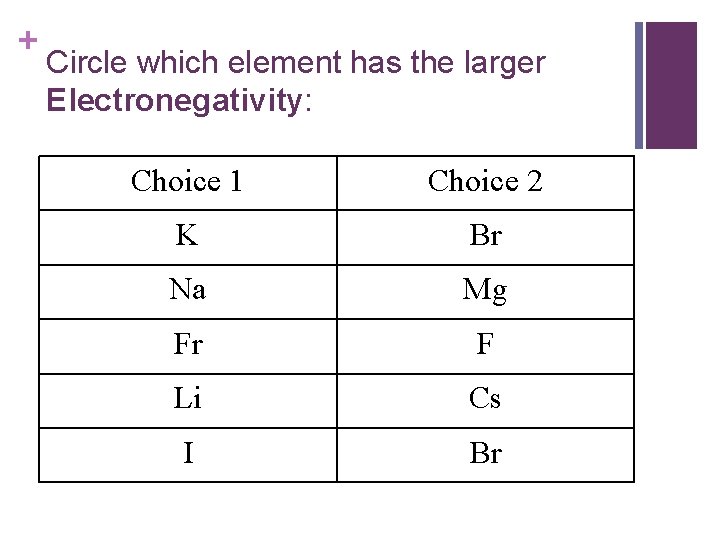
+ Circle which element has the larger Electronegativity: Choice 1 Choice 2 K Br Na Mg Fr F Li Cs I Br
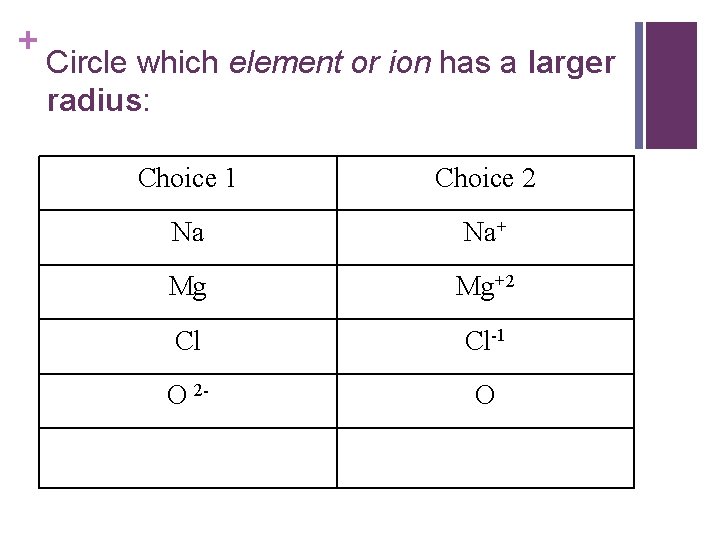
+ Circle which element or ion has a larger radius: Choice 1 Choice 2 Na Na+ Mg Mg+2 Cl Cl-1 O 2 - O
- Slides: 27