The Periodic Table The Ultimate CheatSheet Elementary My

The Periodic Table The Ultimate Cheat-Sheet

Elementary My Dear Watson…. n n n Elements are distinguished by the number of protons Each element has unique properties How are they arranged? Is there a pattern?

The “Original”

Why Do We Need a Periodic Table? n n By 1700, only 13 elements were known The rate of discovery increased in the 18 th century (Davy, Lavoisier, Priestly) But how could scientists know an element was “new? ” Chemists needed a way to organize the elements

How Was It Developed? n In 1829, Dobereiner published a classification system using triads n Triads are groups of 3 elements with similar properties n But, not all elements could be grouped into triads

Lothar Meyer n n In 1864, Lothar Meyer published an early version of the periodic table It contained 28 elements classified into 6 families by their valence (combining power) This was the first time that elements had been grouped and ordered according to their valence. Work on organizing the elements by atomic weight had hitherto been stymied by inaccurate measurements of the atomic weights. 7
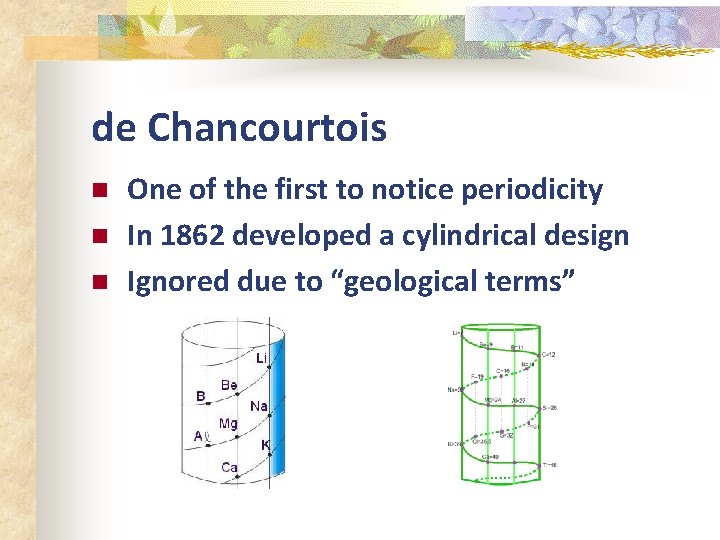
de Chancourtois n n n One of the first to notice periodicity In 1862 developed a cylindrical design Ignored due to “geological terms”
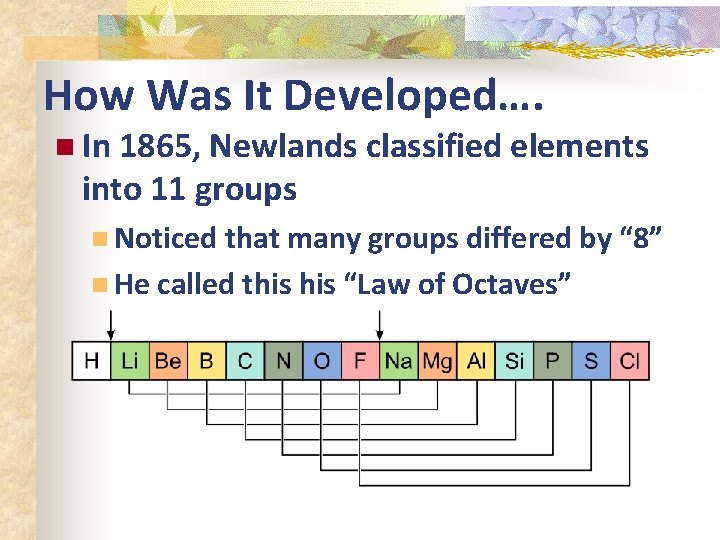
How Was It Developed…. n In 1865, Newlands classified elements into 11 groups n Noticed that many groups differed by “ 8” n He called this “Law of Octaves”

Development n n Other systems were explored… In 1869, Dmitri Mendeleev proposed his periodic table He played “chemical solitaire” on the train n There were 60 elements to organize n 1834 -1907


How Did Mendeleev Do It? n n n He organized the elements by increasing atomic mass and “combining power” He left a space in his table if an element was unknown In time, those spaces were filled in with elements that matched his predictions
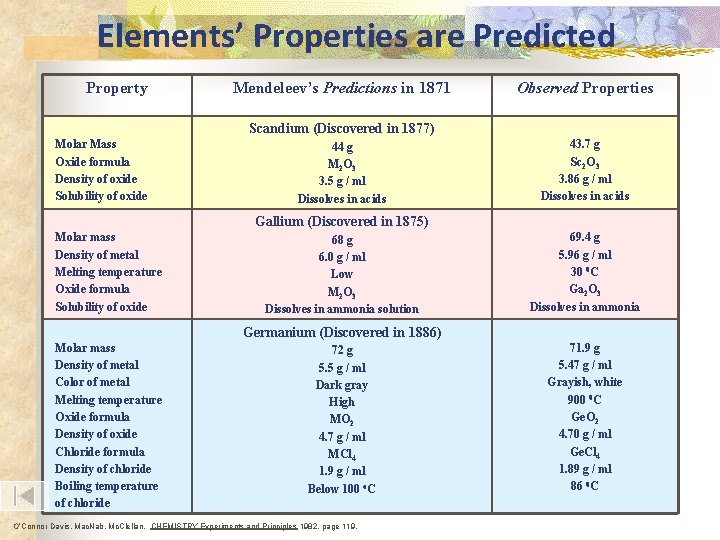
Elements’ Properties are Predicted Property Mendeleev’s Predictions in 1871 Observed Properties Scandium (Discovered in 1877) Molar Mass Oxide formula Density of oxide Solubility of oxide 44 g M 2 O 3 3. 5 g / ml Dissolves in acids 43. 7 g Sc 2 O 3 3. 86 g / ml Dissolves in acids Gallium (Discovered in 1875) Molar mass Density of metal Melting temperature Oxide formula Solubility of oxide 68 g 6. 0 g / ml Low M 2 O 3 Dissolves in ammonia solution 69. 4 g 5. 96 g / ml 30 0 C Ga 2 O 3 Dissolves in ammonia Germanium (Discovered in 1886) Molar mass Density of metal Color of metal Melting temperature Oxide formula Density of oxide Chloride formula Density of chloride Boiling temperature of chloride 72 g 5. 5 g / ml Dark gray High MO 2 4. 7 g / ml MCl 4 1. 9 g / ml Below 100 o. C O’Connor Davis, Mac. Nab, Mc. Clellan, CHEMISTRY Experiments and Principles 1982, page 119, 71. 9 g 5. 47 g / ml Grayish, white 900 0 C Ge. O 2 4. 70 g / ml Ge. Cl 4 1. 89 g / ml 86 0 C

Mendeleev n n Some people consider Meyer and Mendeleev the co-creators of the periodic table Most agree that Mendeleev's accurate prediction of the qualities of what he called ekasilicon (germanium), eka-aluminium (gallium) and eka-boron (scandium) qualifies him for deserving the majority of the credit for studies 14

n n Mendeleev did not know the structure of atoms and that the number of protons was unique for each element Now the periodic table is arranged by increasing atomic number
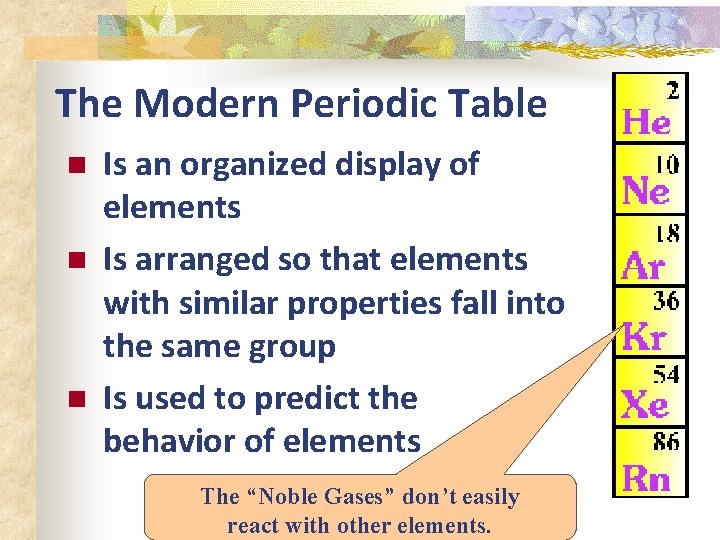
The Modern Periodic Table n n n Is an organized display of elements Is arranged so that elements with similar properties fall into the same group Is used to predict the behavior of elements The “Noble Gases” don’t easily react with other elements.
Rows of the Periodic Table n n Rows of the PT are called “periods. ” All of the elements in a period have the same number of energy levels for their valence (s & p) electrons
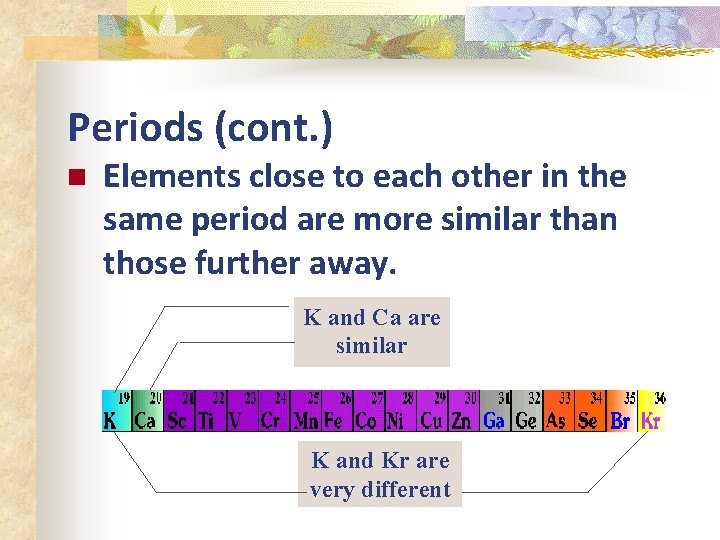
Periods (cont. ) n Elements close to each other in the same period are more similar than those further away. K and Ca are similar K and Kr are very different

So… n n Properties of the elements within a period change as we move across a period from left to right The pattern of properties within a period repeats as we move from one period to the next

The Periodic Law When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties

Regions of the PT
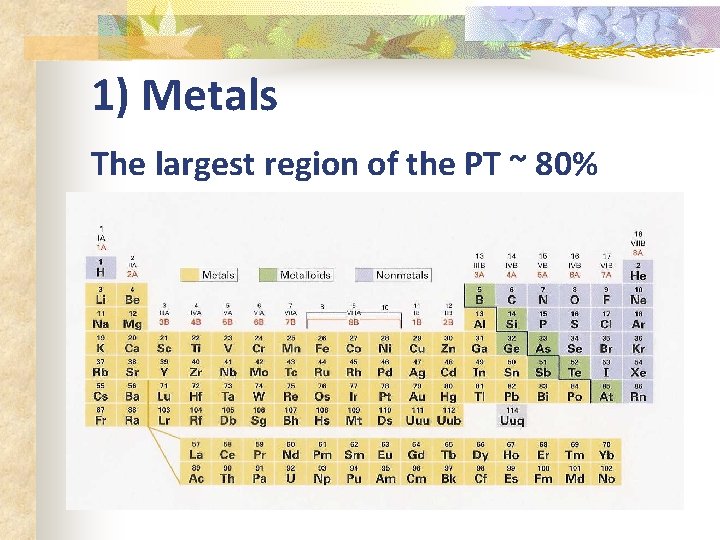
1) Metals The largest region of the PT ~ 80%

Properties of Metals n n Excellent conductors of heat and electricity Usually lustrous, ductile, and malleable. Sodium metal Copper wire Gold charm

2) Nonmetals The second largest region of the PT

Properties of Nonmetals n n Poor conductors of heat and electricity Most are gases or brittle solids at room temperature. Graphite Diamond Chlorine gas
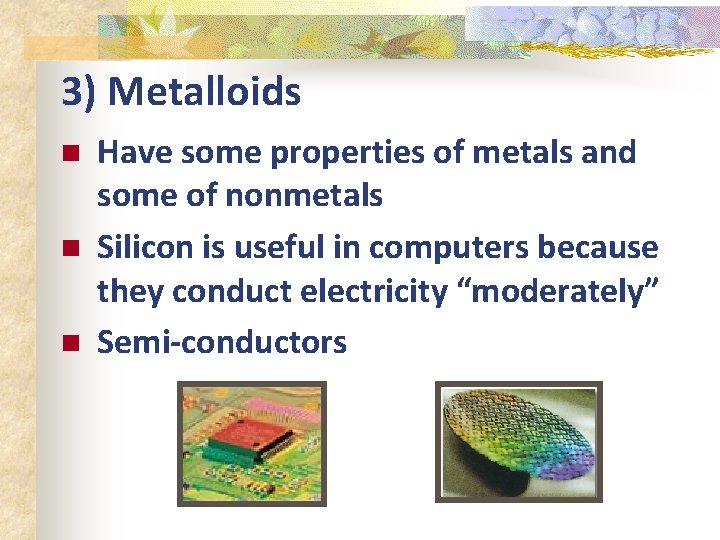
3) Metalloids n n n Have some properties of metals and some of nonmetals Silicon is useful in computers because they conduct electricity “moderately” Semi-conductors

Other Uses of Metalloids n n n Lasers Infrared sensors Alloys Glass products Added Impurities
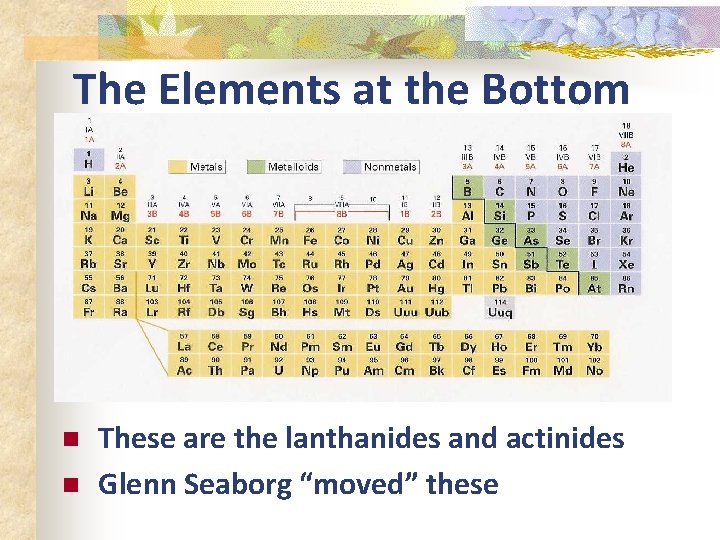
The Elements at the Bottom n n These are the lanthanides and actinides Glenn Seaborg “moved” these

Special Groups of Elements n Group 1 A – the Alkali Metals n n The name alkali comes from the Arabic al aqali, meaning “the ashes. ” Wood ashes are rich in compounds containing sodium and potassium Group 2 A – the Alkaline Earth Metals Group 7 A – the Halogens n Literally means “salt former” 30

The Representative Elements n n These are the elements in Groups 1 A – 7 A They represent a wide range of properties n n n Metals, nonmetals, and metalloids Solids, liquid (Br), and gases The highest level s & p orbitals are NOT filled 31
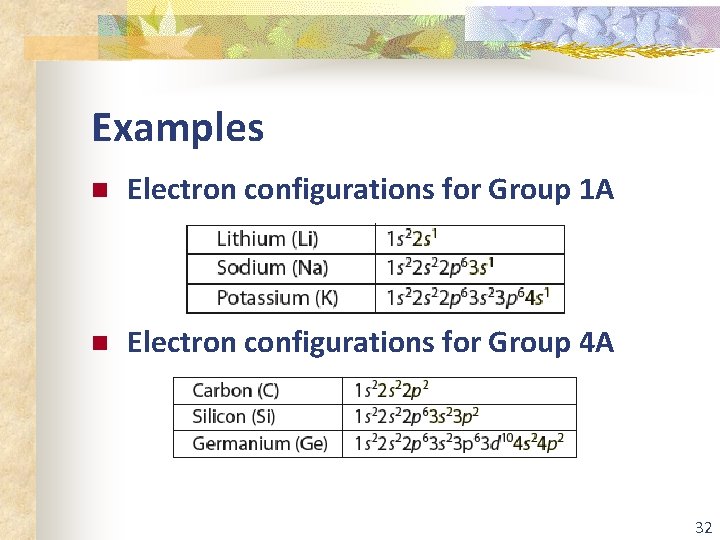
Examples n Electron configurations for Group 1 A n Electron configurations for Group 4 A 32
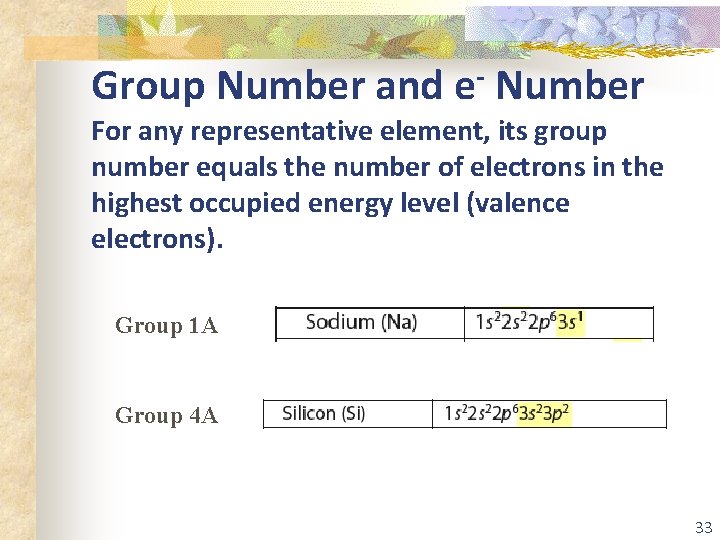
Group Number and e- Number For any representative element, its group number equals the number of electrons in the highest occupied energy level (valence electrons). Group 1 A Group 4 A 33

Noble Gases n n n NOT considered representative elements Unreactive Uses: Krypton is mixed with Argon in fluorescent lights (also to render Superman inert) Neon is used for signs Helium is used in weather and toy balloons

What About The “Ones in the Middle” n n These have different letter designations, depending on the table These are also called n n Transition metals Inner transition metals 35
Transition Metals n n The highest occupied s sublevel and a nearby d sublevel contain electrons These elements are also called d -block elements 36

The Inner Transition Metals n n AKA: the lanthanides and actinides. The highest occupied s sublevel and a nearby f sublevel generally contain electrons. 37
f-orbitals 38
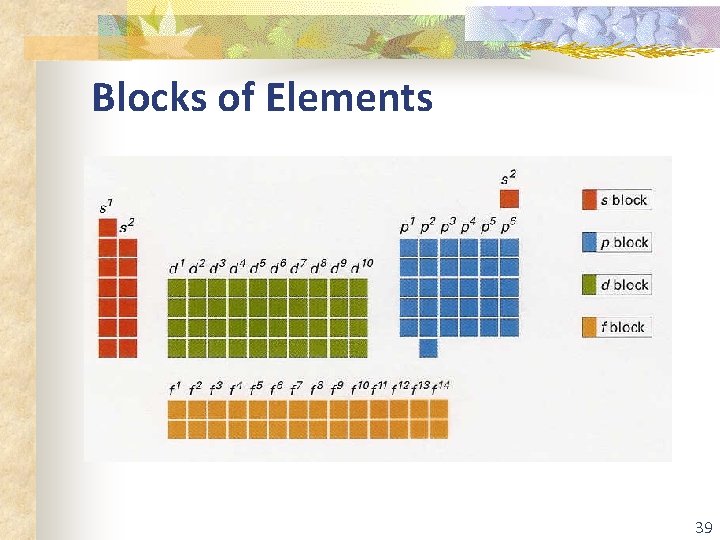
Blocks of Elements 39

Rare Earth Elements n n Scandium, Yttrium, Lanthanum, and Cerium through Lutetium Used in electronics n n n Thulium (Tm) – lasers & x-rays Neodymium (Nd) - magnets Not rare – hard to separate n n Elements very similar Charges are 3+ 40
The Racetrack Design
- Slides: 41