The Periodic Table Review What are the family
The Periodic Table Review
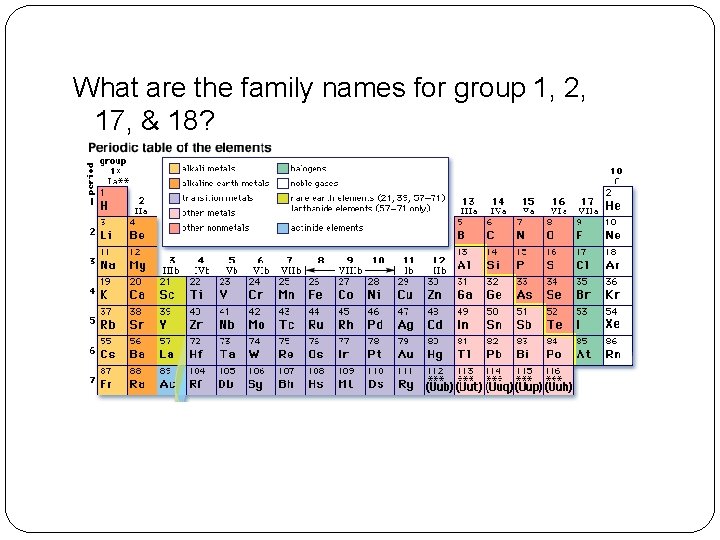
What are the family names for group 1, 2, 17, & 18?
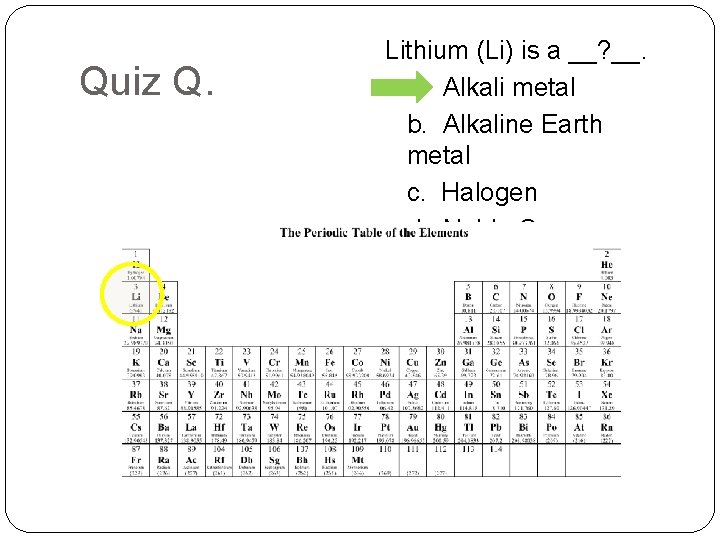
Quiz Q. Lithium (Li) is a __? __. a. Alkali metal b. Alkaline Earth metal c. Halogen d. Noble Gas
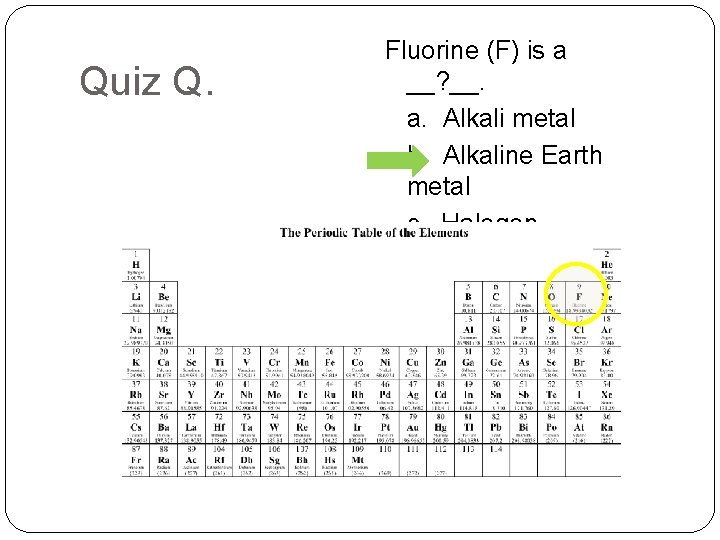
Quiz Q. Fluorine (F) is a __? __. a. Alkali metal b. Alkaline Earth metal c. Halogen d. Noble Gas
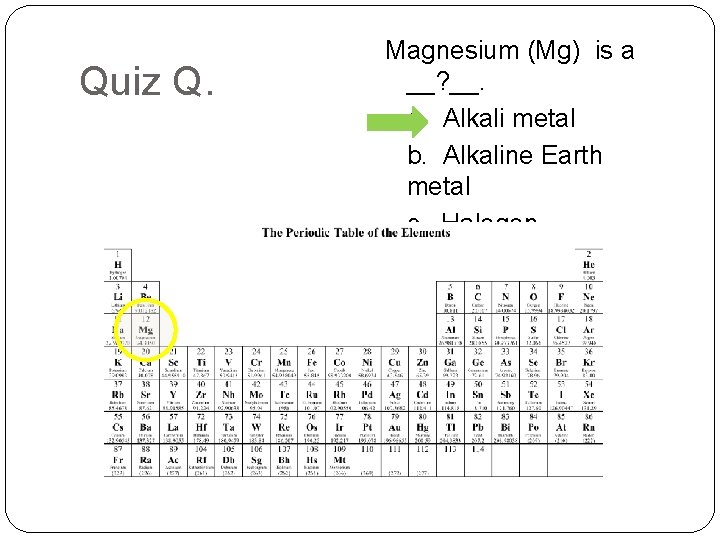
Quiz Q. Magnesium (Mg) is a __? __. a. Alkali metal b. Alkaline Earth metal c. Halogen d. Noble Gas
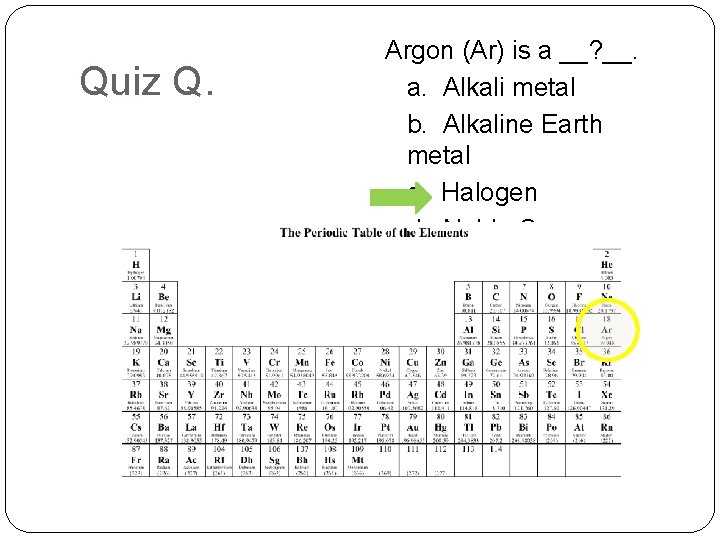
Quiz Q. Argon (Ar) is a __? __. a. Alkali metal b. Alkaline Earth metal c. Halogen d. Noble Gas
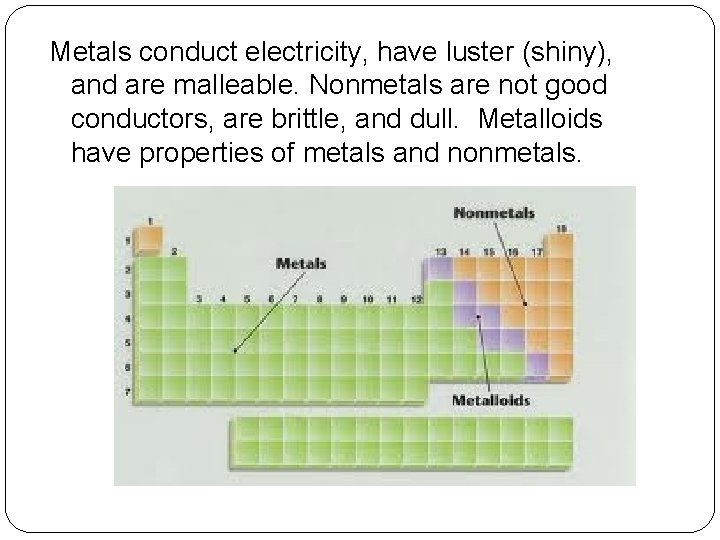
Metals conduct electricity, have luster (shiny), and are malleable. Nonmetals are not good conductors, are brittle, and dull. Metalloids have properties of metals and nonmetals.
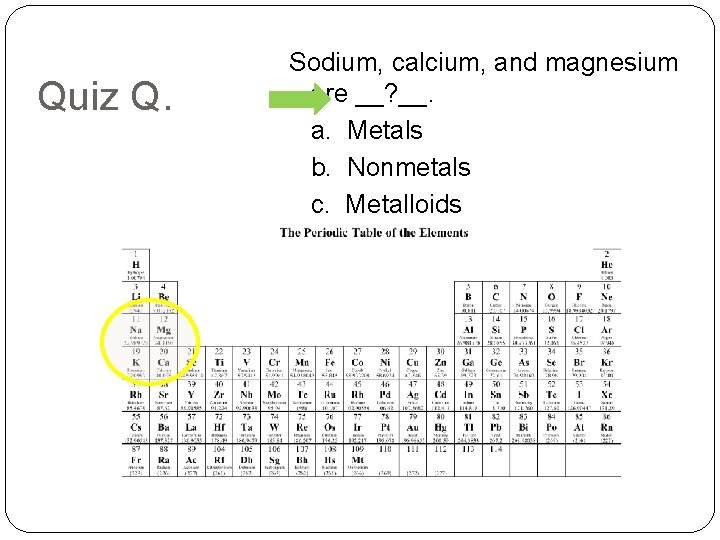
Quiz Q. Sodium, calcium, and magnesium are __? __. a. Metals b. Nonmetals c. Metalloids
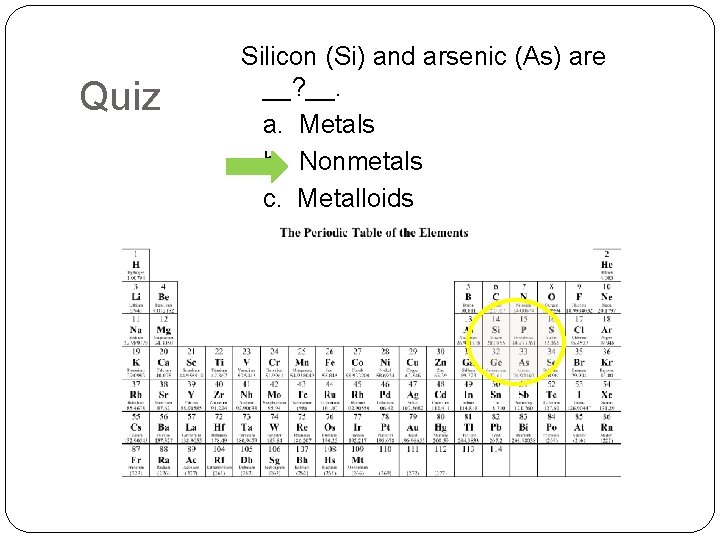
Quiz Silicon (Si) and arsenic (As) are __? __. a. Metals b. Nonmetals c. Metalloids
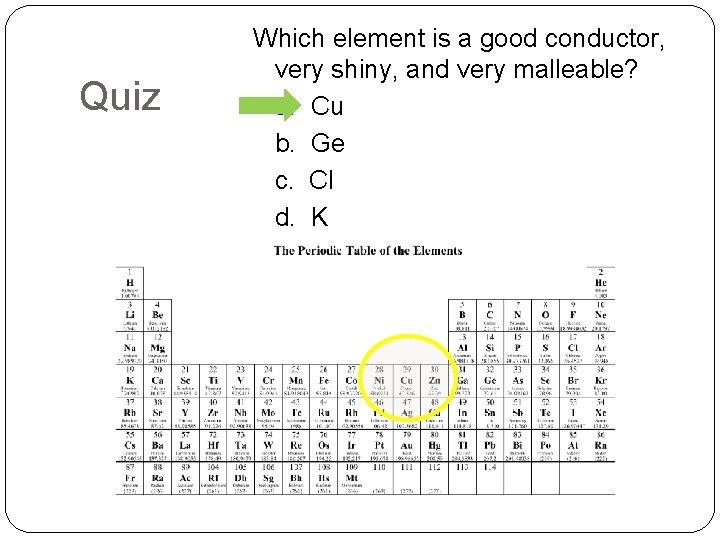
Quiz Which element is a good conductor, very shiny, and very malleable? a. Cu b. Ge c. Cl d. K
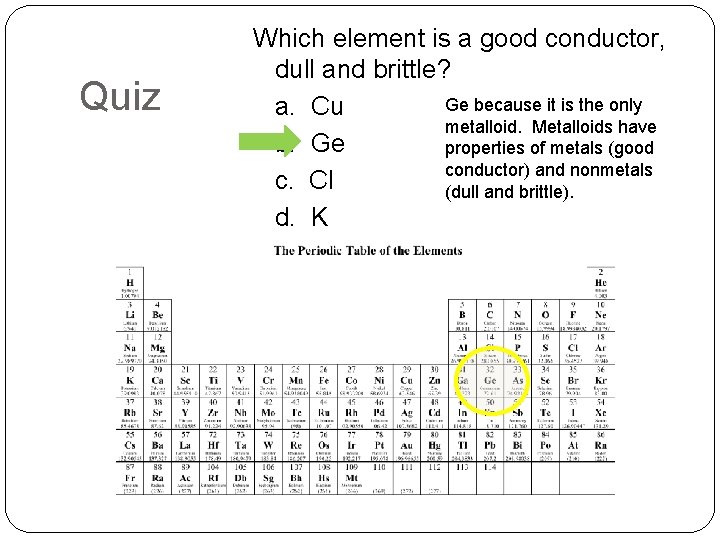
Quiz Which element is a good conductor, dull and brittle? Ge because it is the only a. Cu metalloid. Metalloids have b. Ge properties of metals (good conductor) and nonmetals c. Cl (dull and brittle). d. K
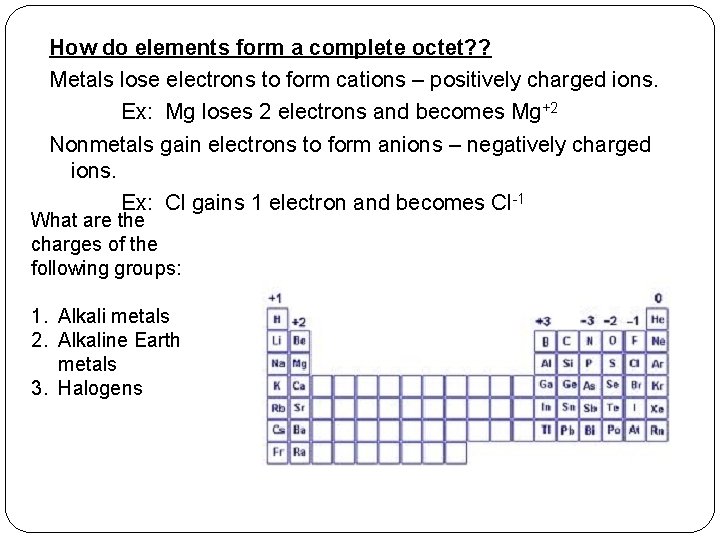
How do elements form a complete octet? ? Metals lose electrons to form cations – positively charged ions. Ex: Mg loses 2 electrons and becomes Mg+2 Nonmetals gain electrons to form anions – negatively charged ions. Ex: Cl gains 1 electron and becomes Cl-1 What are the charges of the following groups: 1. Alkali metals 2. Alkaline Earth metals 3. Halogens
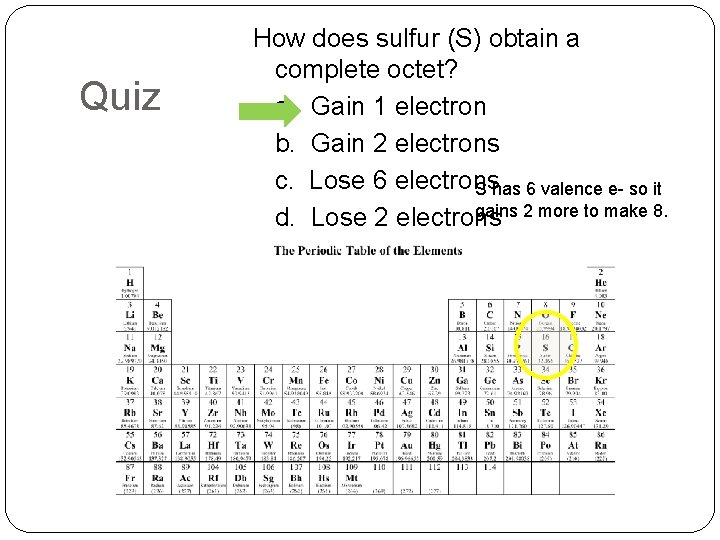
Quiz How does sulfur (S) obtain a complete octet? a. Gain 1 electron b. Gain 2 electrons c. Lose 6 electrons S has 6 valence e- so it gains 2 more to make 8. d. Lose 2 electrons
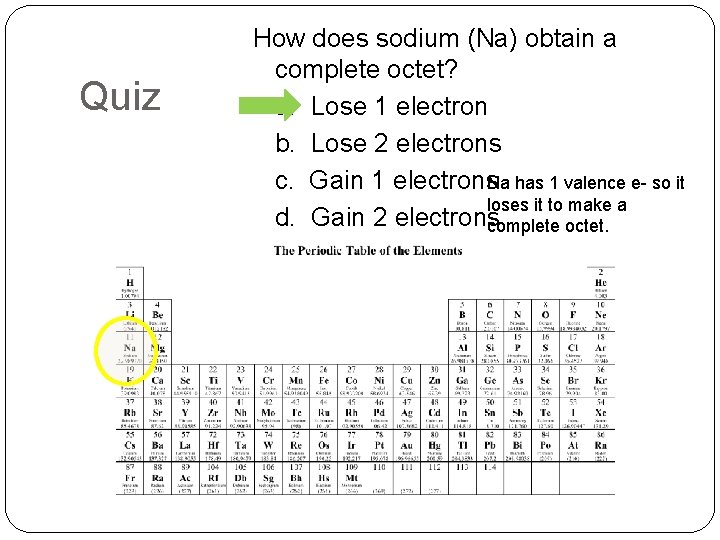
Quiz How does sodium (Na) obtain a complete octet? a. Lose 1 electron b. Lose 2 electrons c. Gain 1 electrons. Na has 1 valence e- so it loses it to make a d. Gain 2 electronscomplete octet.
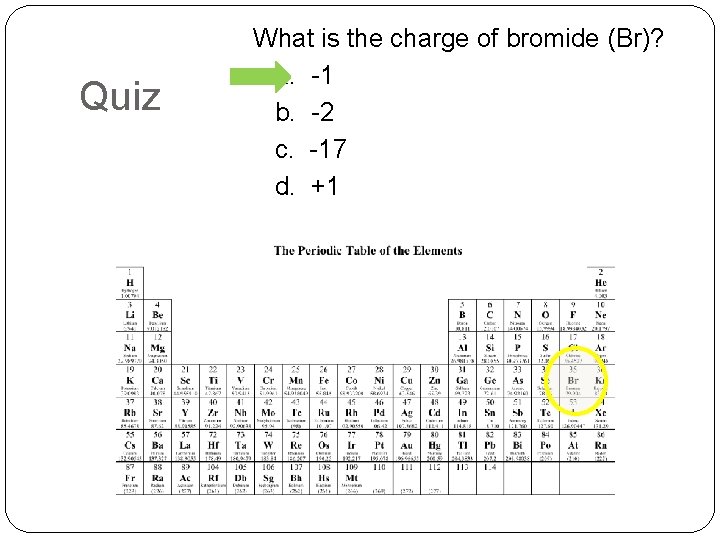
Quiz What is the charge of bromide (Br)? a. -1 b. -2 c. -17 d. +1
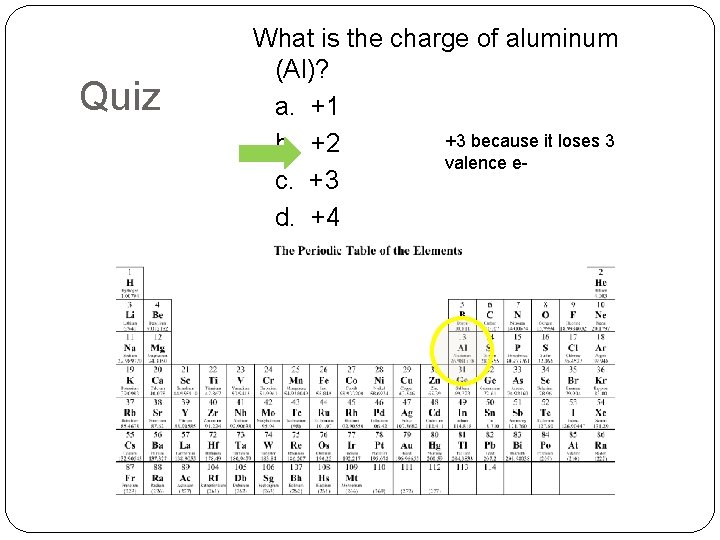
Quiz What is the charge of aluminum (Al)? a. +1 +3 because it loses 3 b. +2 valence ec. +3 d. +4
Quiz Which of the following elements will form a cation? a. Sr b. N c. I d. Ne
Anions are negative because they… Quiz a. Lose electrons. b. Gain electrons. These are all negative.
How do transition metals form a complete octet? ? Transition metals, tin and lead lose electrons to form cations. However, they lose a varying amount of electrons so Roman Numerals must be used to indicate how many electrons were lost. Ex: Copper may lose 1 electron and become Cu+1 or copper may lose 2 electrons and become Cu+2.
Quiz What is the name of Fe+2? a. Iron b. Iron ion The Roman c. Iron (II) ion numeral indicates the charge d. Iron (I) ion
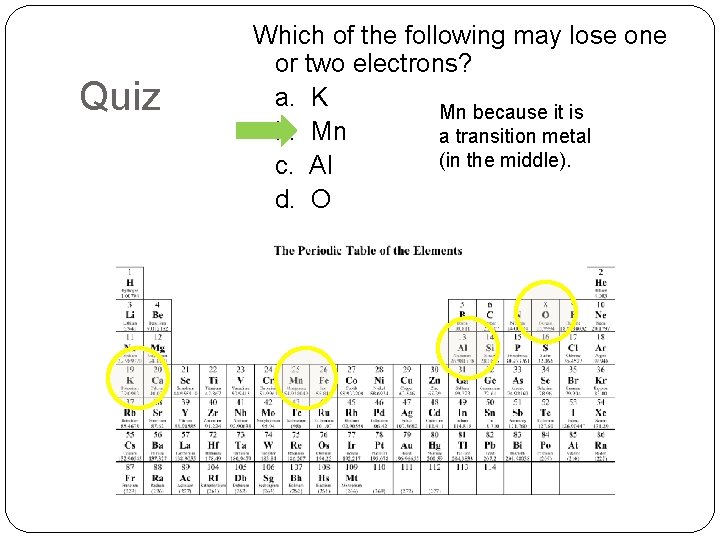
Quiz Which of the following may lose one or two electrons? a. K Mn because it is b. Mn a transition metal (in the middle). c. Al d. O
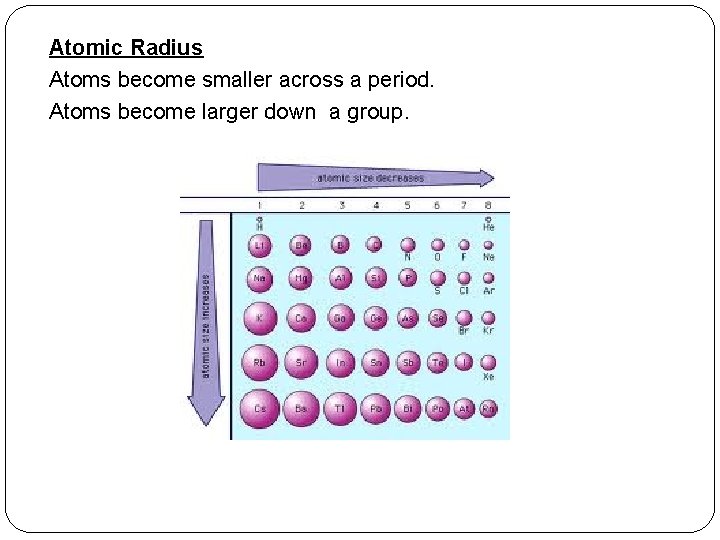
Atomic Radius Atoms become smaller across a period. Atoms become larger down a group.
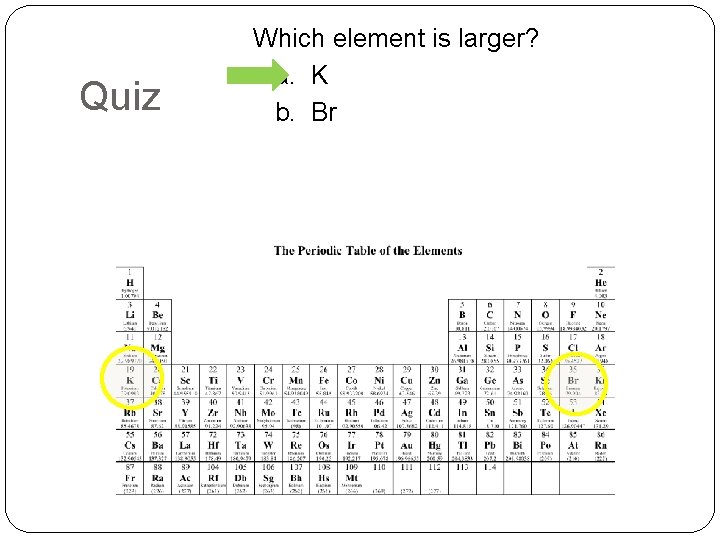
Quiz Which element is larger? a. K b. Br
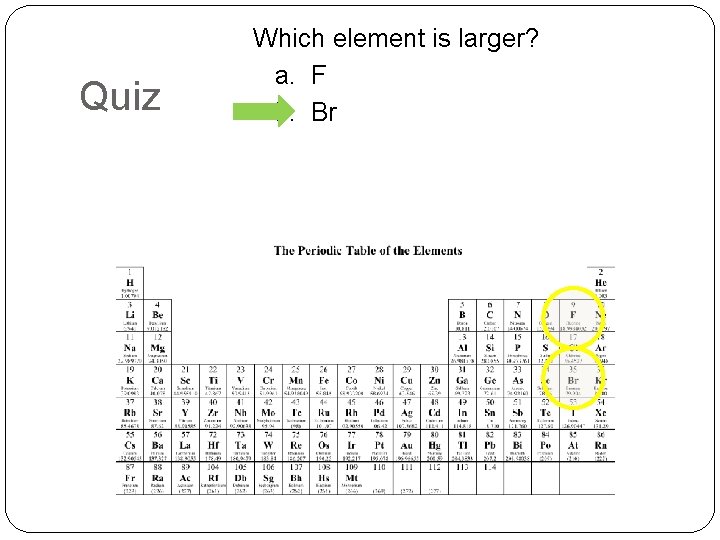
Quiz Which element is larger? a. F b. Br
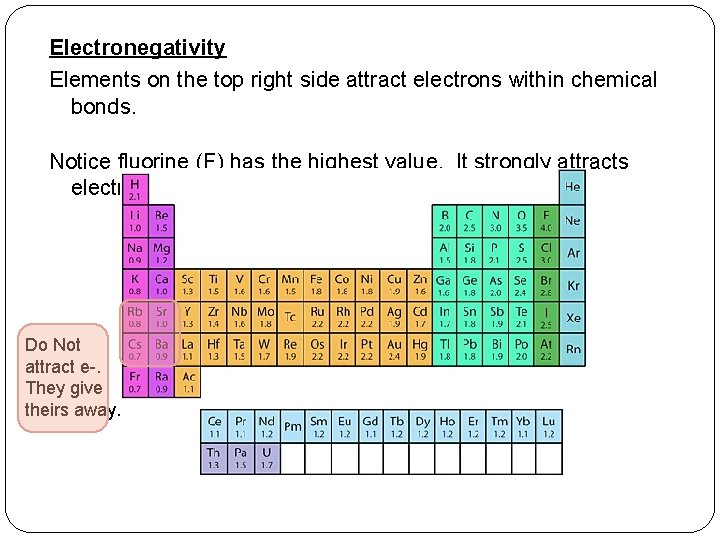
Electronegativity Elements on the top right side attract electrons within chemical bonds. Notice fluorine (F) has the highest value. It strongly attracts electrons. Do Not attract e-. They give theirs away.
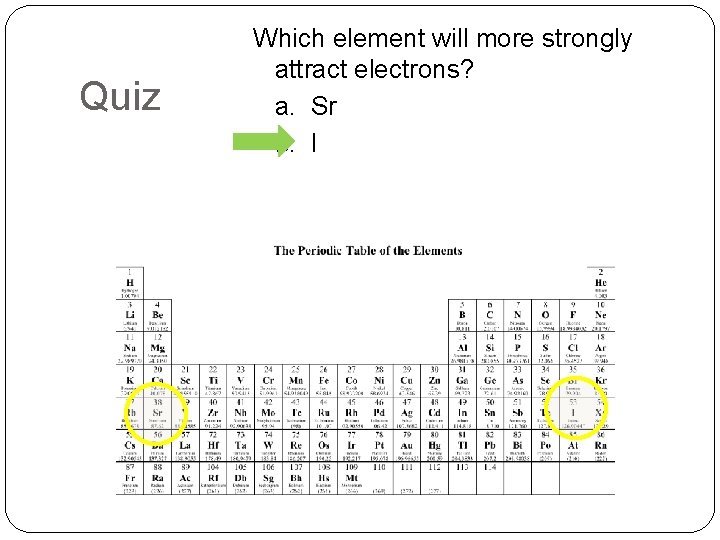
Quiz Which element will more strongly attract electrons? a. Sr b. I
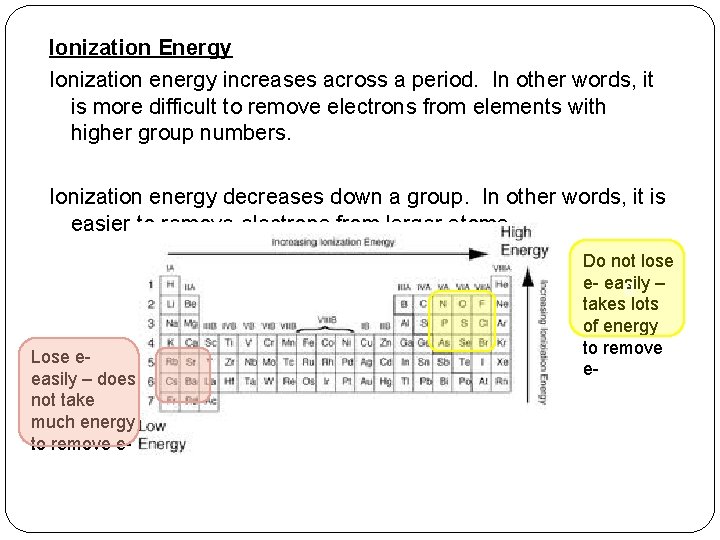
Ionization Energy Ionization energy increases across a period. In other words, it is more difficult to remove electrons from elements with higher group numbers. Ionization energy decreases down a group. In other words, it is easier to remove electrons from larger atoms. Lose eeasily – does not take much energy to remove e- Do not lose e- easily 0 – takes lots of energy to remove e-
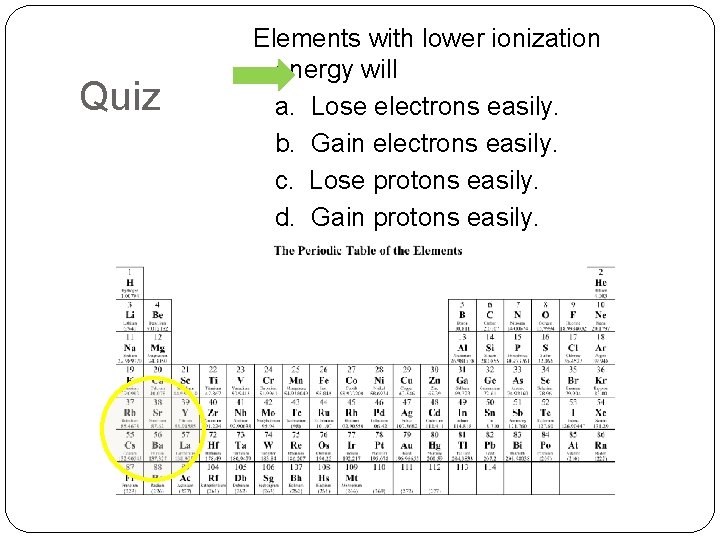
Quiz Elements with lower ionization energy will a. Lose electrons easily. b. Gain electrons easily. c. Lose protons easily. d. Gain protons easily.
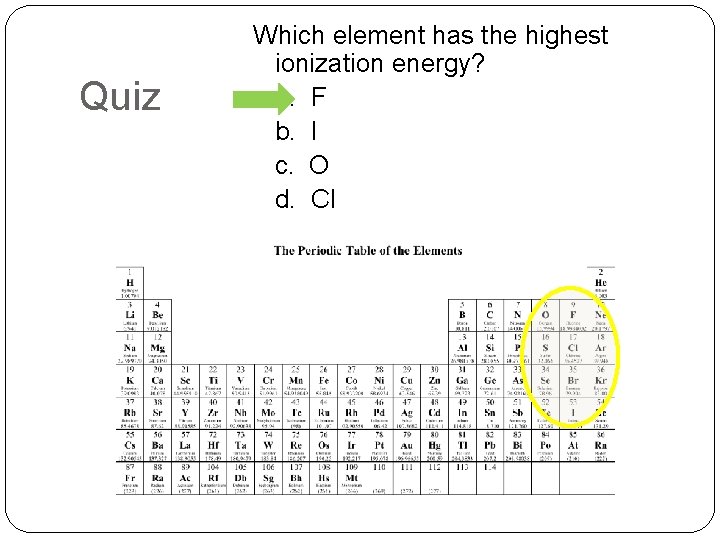
Quiz Which element has the highest ionization energy? a. F b. I c. O d. Cl
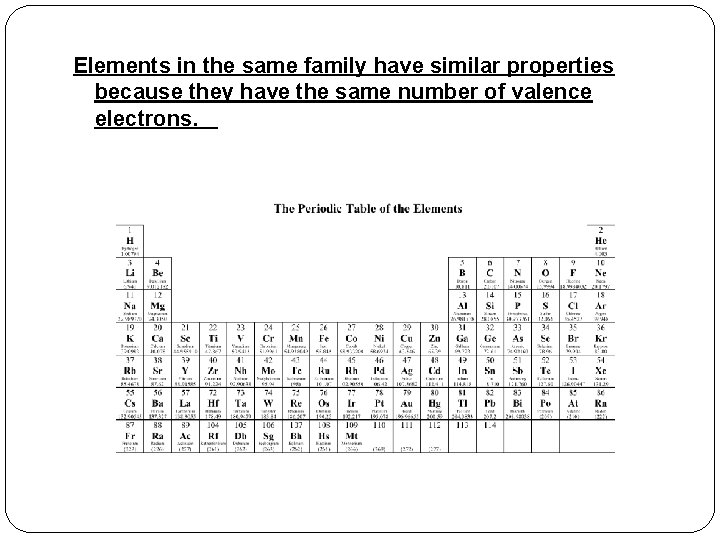
Elements in the same family have similar properties because they have the same number of valence electrons.
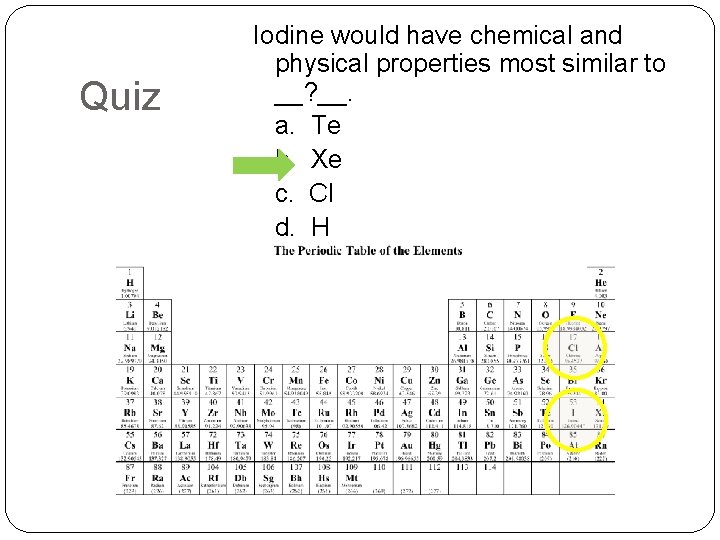
Quiz Iodine would have chemical and physical properties most similar to __? __. a. Te b. Xe c. Cl d. H
Quiz What is the name of Ca+2? a. Calcium Add the word ion to b. Calcium ion any cation c. Calciumide d. Calcium (II) ion
Quiz What is the name of O-2? a. Oxygen ion Change the b. Oxygen (II) ion ending of anions to –ide c. Oxide d. Oxygenide
Quiz What is the symbol for phosphide? a. P+3 All elements in group 15 form b. P+5 ions with a -3 -3 c. P charge d. P-8 +1 +2 +3 1 -3 -2 -
Quiz Which of the following ion symbols is correct? a. Cl+1 b. Ba+2 c. Li-1 d. Al-3 +1 +2 +3 1 -3 -2 -
Quiz Lose e- easiest – very reactive What is the most reactive family of metals? a. Halogens b. Noble gases c. Alkaline earth metals d. Alkali metals
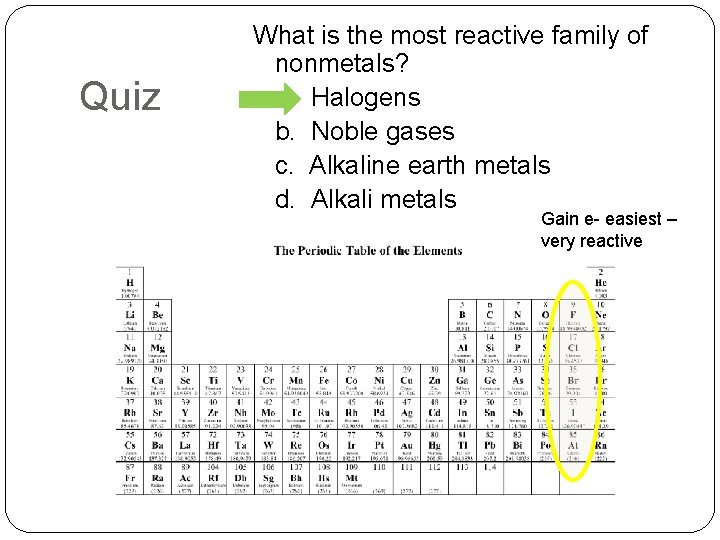
Quiz What is the most reactive family of nonmetals? a. Halogens b. Noble gases c. Alkaline earth metals d. Alkali metals Gain e- easiest – very reactive
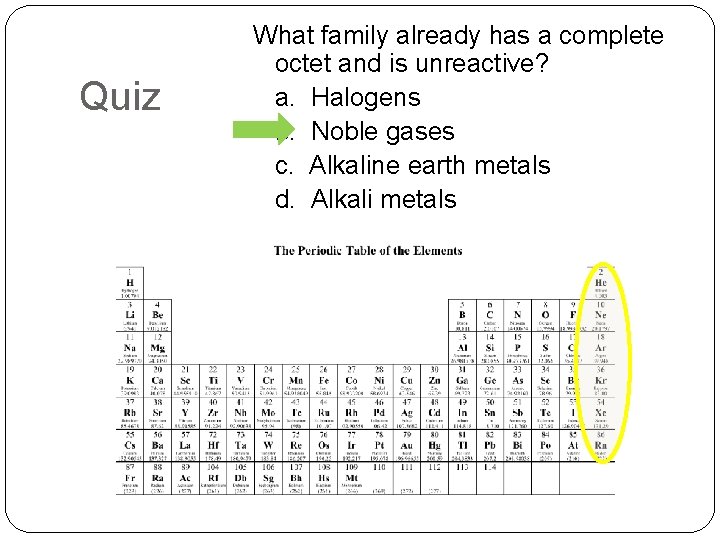
Quiz What family already has a complete octet and is unreactive? a. Halogens b. Noble gases c. Alkaline earth metals d. Alkali metals
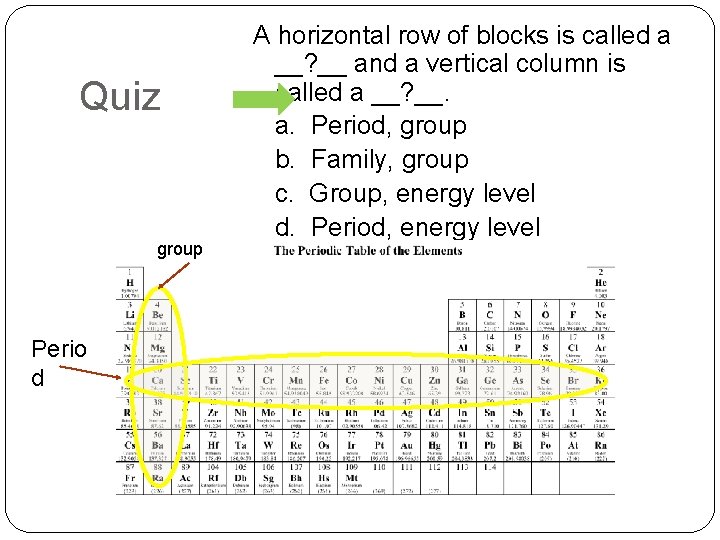
Quiz group Perio d A horizontal row of blocks is called a __? __ and a vertical column is called a __? __. a. Period, group b. Family, group c. Group, energy level d. Period, energy level
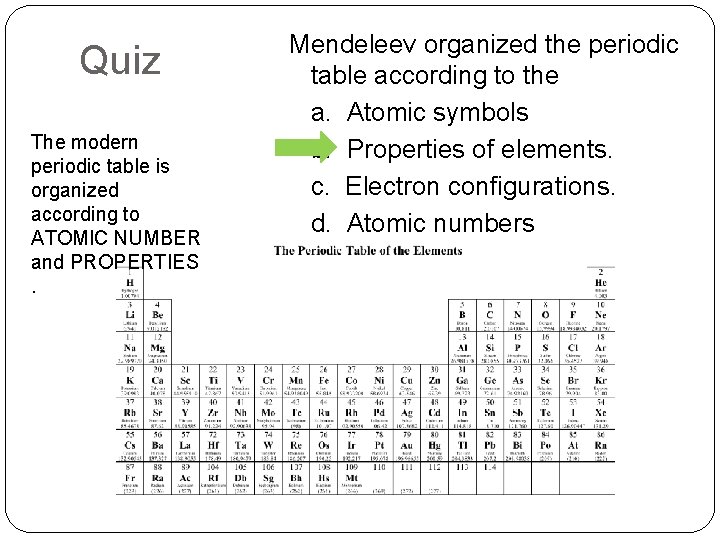
Quiz The modern periodic table is organized according to ATOMIC NUMBER and PROPERTIES. Mendeleev organized the periodic table according to the a. Atomic symbols b. Properties of elements. c. Electron configurations. d. Atomic numbers
- Slides: 40