The Periodic Table Periodic Table What is it
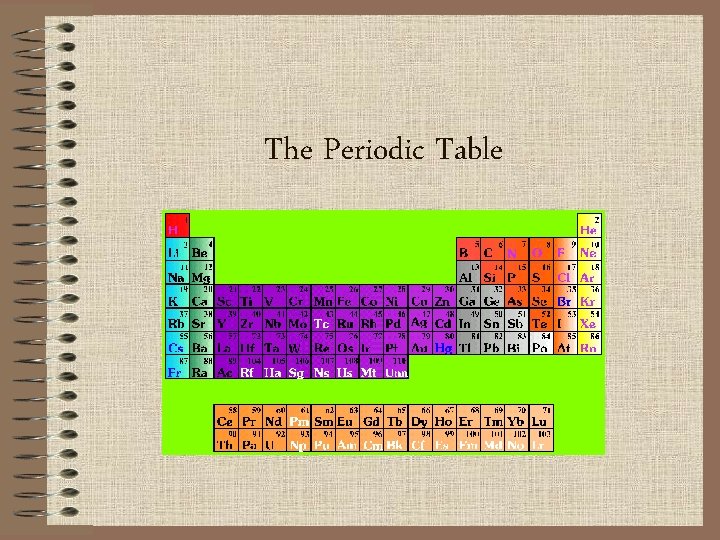
The Periodic Table
Periodic Table: What is it? • An arrangement of elements according to repeated changes in properties

Dimitri Mendeleev • • Worked in the late 1800’s Arranged the elements by increasing atomic mass Accurately predicted new elements based on properties (Ex. Ge) Problem: Co, Ni and Te, I atomic masses don’t increase!

Henry Moseley • Worked in the early 1900’s (1913) • Created the modern version of the periodic table • Arranged the elements by increasing atomic number (PROTONS!)
GROUPS • Vertical columns on the periodic table • Elements is the same group have similar properties because they have the same number of valence electrons! – – – – Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = 6 valence electrons Group 17 = 7 valence electrons Group 18 = 8 valence electrons
GROUPS • In order to satisfy the Octet Rule, Groups 1, 2, and 13 tend to give up their electrons when reacting with other elements. • In contrast, Groups 14, 15, 16, and 17 tend to accept electrons when reacting with other elements to satisfy the Octet Rule.

Octet Rule • Octet Rule – states that atoms tend to combine in such a way that they each have eight electrons in their valence shells, giving them the same electron configuration as a noble gas. • An atom is chemically stable if its outer energy level is completely filled with electrons.
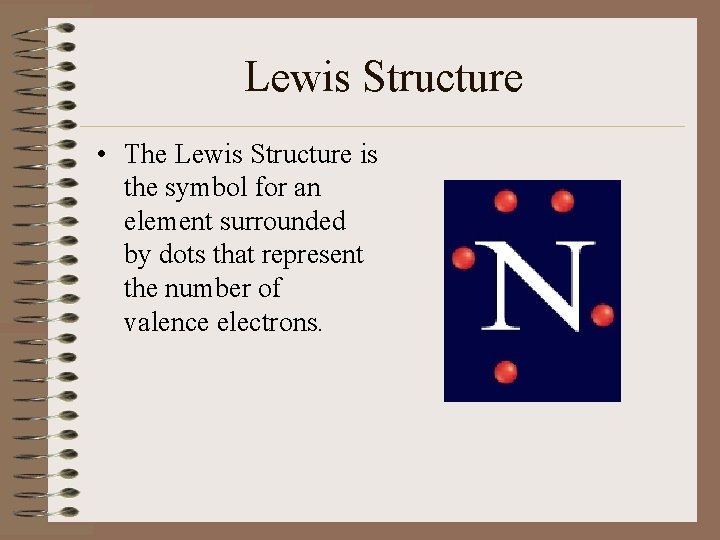
Lewis Structure • The Lewis Structure is the symbol for an element surrounded by dots that represent the number of valence electrons.
Periods • Horizontal rows on the periodic table • Periods have the same number of energy levels! • They do NOT necessarily have similar properties!
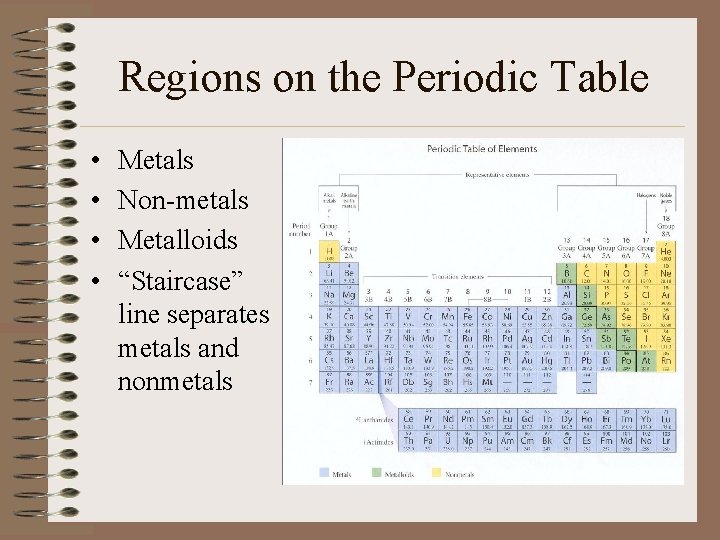
Regions on the Periodic Table • • Metals Non-metals Metalloids “Staircase” line separates metals and nonmetals
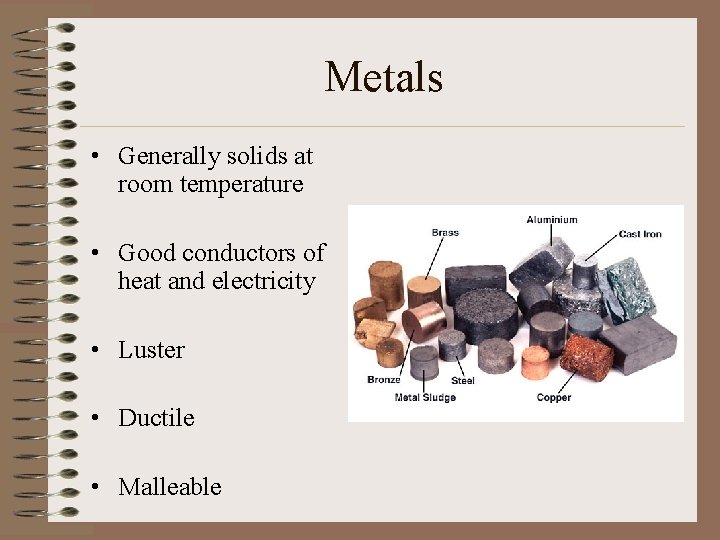
Metals • Generally solids at room temperature • Good conductors of heat and electricity • Luster • Ductile • Malleable

Metals cont. • malleable - the ability to be hammered or rolled into sheets. • ductile - the ability to be drawn into a wire. • metallic bonding - positively charged metallic ions are surrounded by a cloud of electrons

Metals cont. • Mercury is a silverywhite poisonous metallic element, liquid at room temperature and used in thermometers, barometers, vapor lamps, and batteries and in the preparation of chemical pesticides.

Non-Metals • Mostly gases or brittle solids at room temperature • Poor conductors of heat and electricity • Not ductile or malleable

Non-Metals cont. • Bromine is a heavy, reddish-brown liquid. It is the only nonmetallic element that is liquid at room temperature.

Metalloids • Have properties of both metals and non-metals • Semiconductors

Alkali Metals • • • Group 1 – one valence electron Most reactive of metals Do not occur freely in nature Soft, can be cut with a knife All are malleable, ductile, and are good conductors of heat and electricity. • Highly reactive in air and water • Stored in kerosene or mineral oil • Na and K needed to stay healthy

Alkaline Earth Metals • • • All are silver-colored Group 2 – two valence electron Harder and less reactive than Group 1 Still very reactive Not found naturally in elemental form

Alkaline Earth Metal Uses • Mg – – Fireworks Cars, planes, spacecraft Ladders, baseball + softball bats Chlorophyll – contains Mg cmpd that enables plants to make food • Ca – marble statues + some countertops – Humans need for bones • Ba – diagnose digestive disorders • Ra – radioactive + used to treat breast cancer

Transition Elements • • Groups 3 – 12 Often occur in nature as free elements Form brightly colored compounds Ductile, malleable, & conductors of electricity and heat • Several stable oxidation states • High boiling and melting points

Transition Elements cont. • Used as catalysis • Au and Ag are commonly used in jewelry • Fe, Co, and Ni (Iron Triad) are the only elements known to produce a magnetic field. • Cu, Ag, & Au are used to make valuable metal coins
Inner Transition Elements • Lanthanides – Numbers 58 – 71 – Burn easily in Oxygen and react violently with nonmetals. – Used in lasers and magnets. • Actinides – – Numbers 90 – 103 All radioactive + unstable Decay into more stable elements Uranium – used in nuclear reactors

Noble Gases • • • Group 18 – exist as isolated, stable atoms Mostly non-reactive & stable Complete valence shell Satisfies the Octet Rule (except He) All are gases at room temperature Ne + Ar + Kr – used in lights

Halogens • Group 17 • Cl – swimming pools, water purification • Salts – compounds between halogens and metals • I – essential for diet – hormone production of thyroxin to prevent goiter – sublimes when heated – obtained from seawater

Other Families • Boron Group – Group # 13 • Carbon Group – Group #14 • Nitrogen Group – Group #15 • Oxygen Group – Group #16

Transuranium Elements • Elements having more than 92 protons, the atomic number for uranium • All synthetic • All unstable • Many disintegrate quickly

Vocabulary • Diatomic molecule consist of two atoms of the same element in a covalent bond. • Salts are compound formed when an acid combines with a base in a neutralization reaction. • Allotropes are different forms of the same element, have different molecular structure. Diamond Graphite
Vocabulary cont. • Radioactive element is one in which the nucleus breaks down and gives off particles and energy. • Semiconductors are elements that conduct electric current under certain conditions • Sublimation is the process by which a solid changes directly to a vapor without forming a liquid.

Vocabulary cont. • Isotopes are atoms of the same element that have different number of neutrons.
- Slides: 29